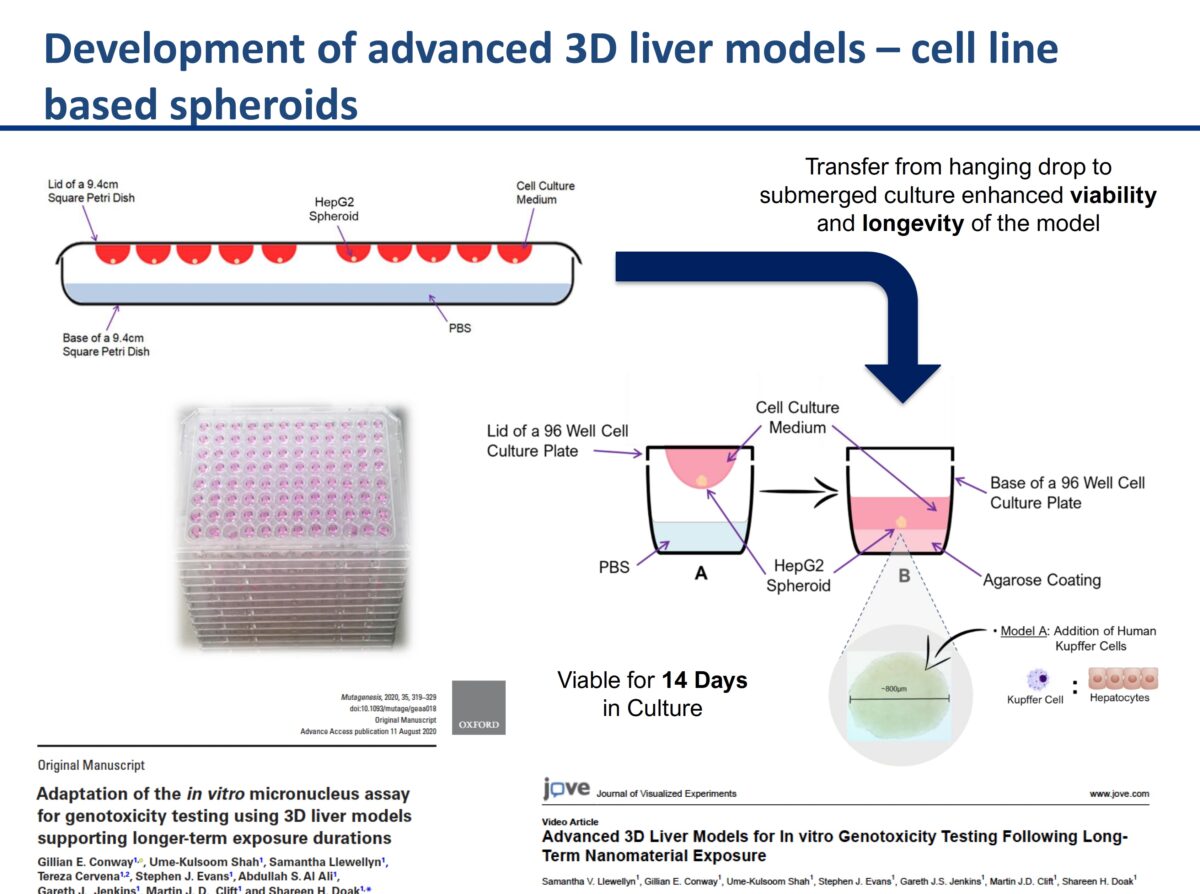
It was an honor to have Professor Shareen Doak with us to discuss her groundbreaking research. Her research on New Approach Methodologies (NAMs) and overcoming limitations of OECD Test Guidelines for nanomaterials (NMs) testing is leading edge brought up a lot of great questions. The link to the recording is below. What you’ll learn: The need to adapt existing …
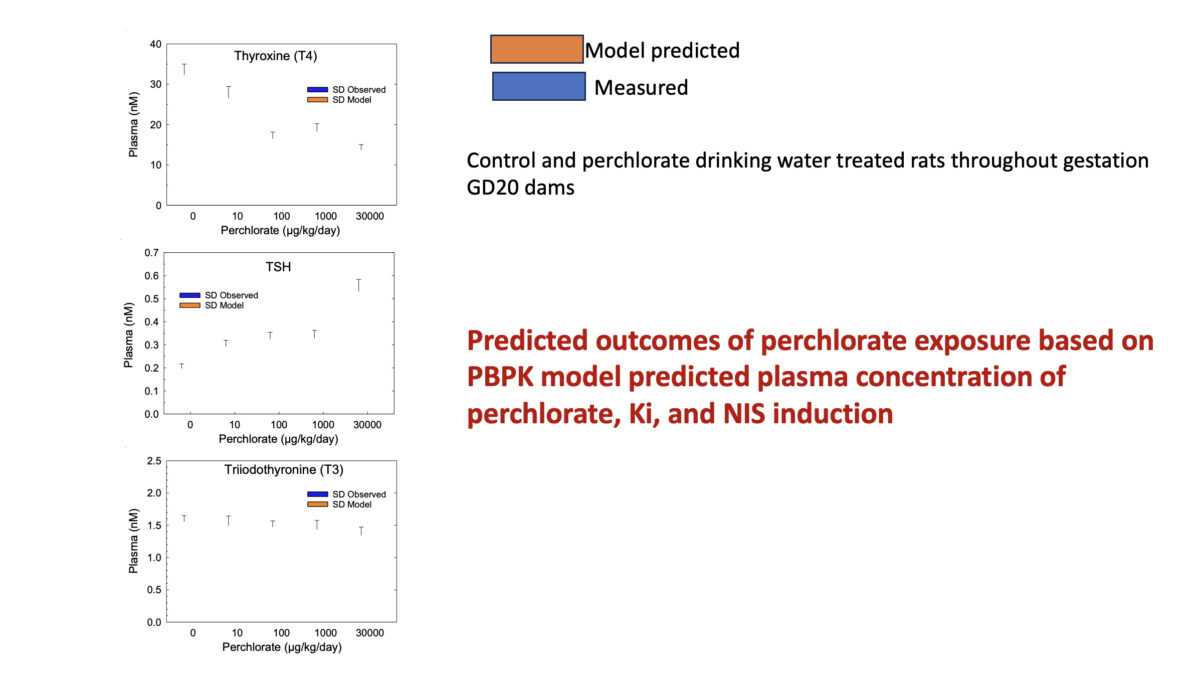
Towards Translating In Vitro Measures Of Thyroid Hormone System Disruption To In Vivo Responses In The Pregnant Rat Via A Biologically Based Dose Response (BBDR) Model
We are excited to present our first recorded installment for our 2024 webinar series. Jeff Fisher, PhD, ScitoVation’s Senior Science Fellow, will be speaking about thyroid disruption and IVIVE based on his latest publication. ScitoVation scientists continue advancing the benefits of IVIVE modeling. What you’ll learn: Thyroid modeling demonstrating IVIVE for a molecular initiating event determined in vitro How to …
Generic Pharmacokinetic Models for Mother-to-Offspring Transfer of Chemicals
Our speaker is Dustin Kapraun, PhD, physical scientist with the Center for Public Health and Environmental Assessment (CPHEA) in the Office of Research and Development (ORD) at the U.S. Environmental Protection Agency (EPA). In developing human health risk assessments, it is important to consider pregnant women, developing fetuses, and nursing children because chemical exposures experienced by these groups can lead …
ToxAIcology – the future of toxicology is AI
Artificial Intelligence (AI) is impacting all types of work, including toxicology. Dr Hartung will discuss how to move toxicology to a more holistic and integrated paradigm using AI and how AI plays a role in setting the direction of “Toxicology for the 21st Century 2.0” in future decades. What you’ll learn: Sources of Big Data for toxicology and various AI …
Physiologically based pharmacokinetic modeling as a data analysis and study design tool – case studies in nonlinear pharmacokinetics
We just had a wonderful talk from Dr. Dan Hoer. Dr Hoer is a physical scientist with the US Environmental Protection Agency, Office of Pesticide Programs. His talk will focus on non-linear PBPK modeling, and the advantages offered by this approach. What you’ll learn: Important advantages of PBPK modeling over statistical methods that are often restricted to bimodal distinctions of …
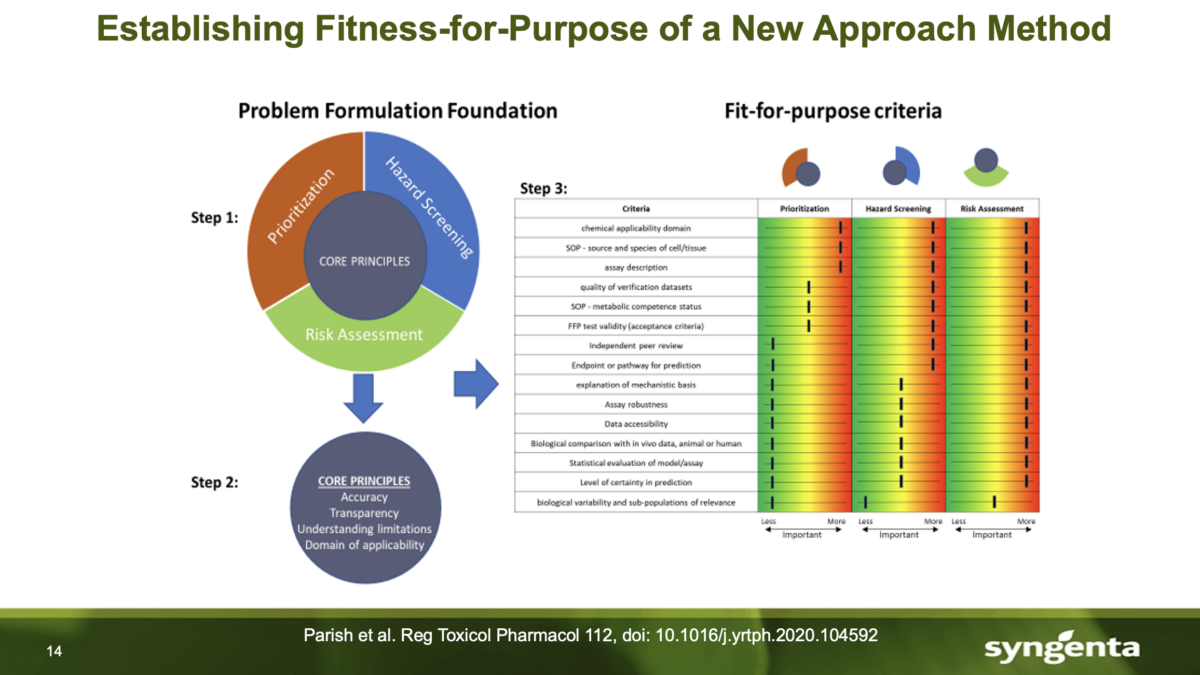
(R)Evolution in Validation: Establishing Scientific Confidence in NAMs
Nicole Kleinstreuer, PhD, the director of the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) and the executive director of the congressionally mandated Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). spoke on July 18th, 2023. Dr. Kleinstreuer discussed a topic very important to our industry; a framework comprising five essential elements to establish scientific …
A Source to Outcome Approach for Inhalation Risk Assessment: The Importance of Collaboration
Doug Wolf, DVM, PhD, discusses the creation and use of the Souce-to-Outcome framework, a new NAM incorporating a novel mathematical procedure developed to estimate the human equivalent concentration (HEC) for inhalation risk assessment based upon the relevant aerosol characterization, respiratory dosimetry modeling, and endpoints derived from an in vitro assay using human respiratory epithelial tissue. What you’ll learn: Introduction to …
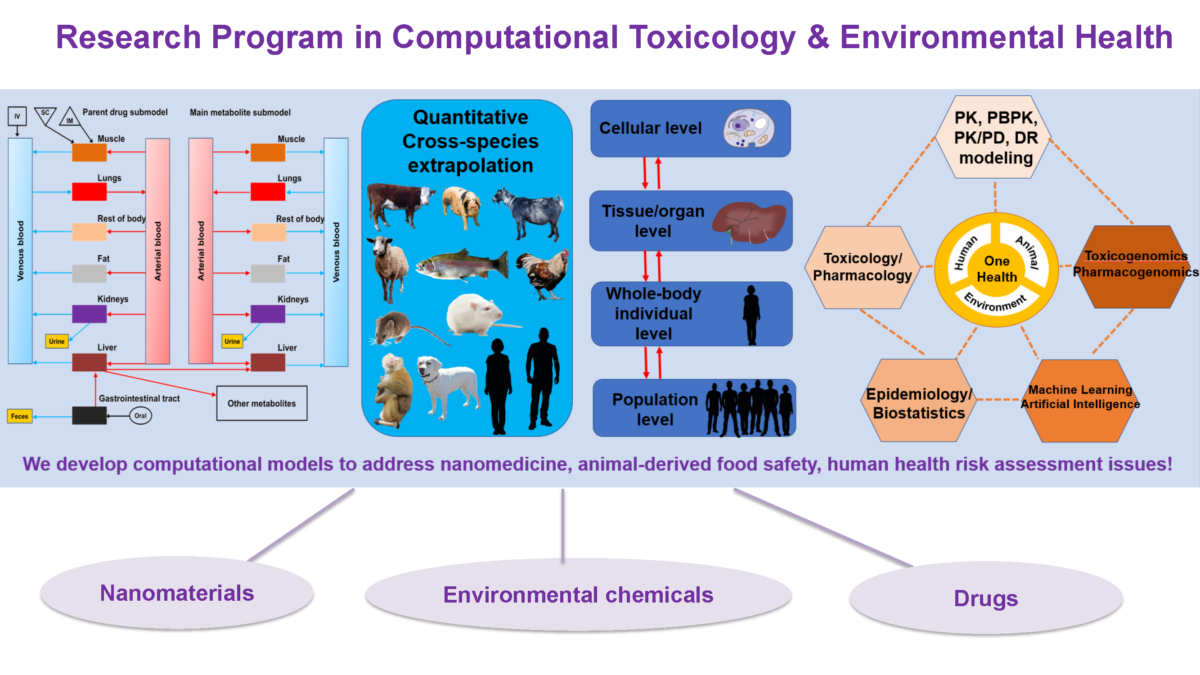
Applications of Physiologically Based Pharmacokinetic (PBPK) Modeling in Nanomedicine, Food Safety and Human Health Risk Assessment and Roles of Artificial Intelligence (AI) Approaches in these Areas
In this presentation, Dr. Zhoumeng Lin introduces how we develop physiologically based pharmacokinetic (PBPK) models of drugs, environmental chemicals and nanoparticles for applications in nanomedicine, food safety, and human health risk assessments. He presents his recent studies to illustrate each application. Dr. Zhoumeng Linalso introduces a new physiological parameter database for PBPK modeling in food-producing animals, including cattle, swine, sheep, …
The Need for Increased Maturity in the Risk Assessment Ecosystem
by Dr. Jean Orelien Last year, the US EPA declared they would phase away from animal testing by 2035. Reaching this milestone will require the maturity of the risk assessment ecosystem. By maturity, I mean new roles need to be assumed along with improved interactions and (stronger) bonds within and between group as well as new entities. In this post, I share a …
- Page 1 of 2
- 1
- 2