Liver toxicity
Human hepatocyte models for safety assessment screening and to accelerate drug discovery
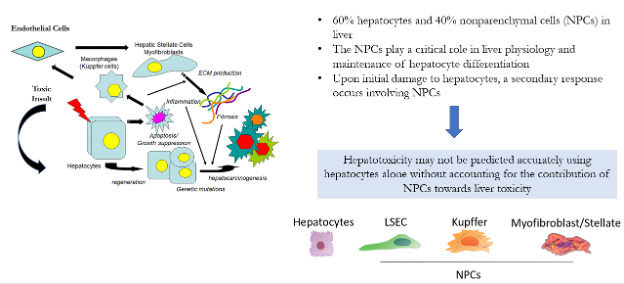
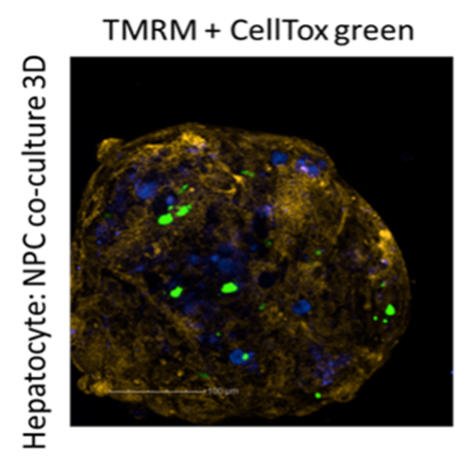
ScitoVation scientists use human hepatocyte models, Primary Human Hepatocytes (PHH), HepaRG™ cells, and the HepG2 cell line for rapid assessments of hepatotoxicity. More recently, ScitoVation scientist have developed a spheroid-based PHH co-cocultured with nonparenchymal cells (NPCs) hepatocytes test system that recapitulates liver functions (Fig 1). Hepatocytes models express Phase I and phase II metabolizing enzyme systems expressed in normal liver, retain intracellular compartmentalization, and can be used to assess xenobiotic-induced enzyme induction. Human hepatocyte models are widely used in investigational toxicology to assess mode-of-action (MOA), xenobiotic biotransformation and hepatotoxicity studies.
ScitoVation offers hepatocyte based in vitro assay systems for safety assessment screening of chemicals and pre-clinical candidates

- Prioritization of new chemicals and pre-clinical candidates to minimize attrition going forward in the pipeline
- Informed go forward or stop decision making
- comparative analysis or ranking/grouping chemicals based on apical measures of toxicity and/or toxicogenomics based benchmark dose (BMD) calculations
Rat hepatocyte and non-parenchymal cell based models
ScitoVation has develop 2D and 3D rat hepatic in vitro models that can be used for safety assessment in lieu of traditional in vivo toxicity studies. These models represent a spectrum of biological and cellular complexity that are fit for the purpose of determining the effects of compounds on endpoints including cellular viability, proliferation, gene expression, metabolism, and the transcriptome. In light of the Environmental Protection Agency’s September 10, 2019, memo committing to the end of mammal in-life testing by 2035, these models can be used for : 1) validation to ensure that new approach methodologies (NAMs) are equivalent to or better than the animal tests replaced and 2) demonstration that NAMs are applicable for use in risk assessment and that new decision-making approaches are as protective of human health and the environment as existing approaches.