Pharma
Determine Safety Early and Efficiently
ScitoVation assists pharma clients within the drug-hunting discovery stages to efficiently identify lead compounds (screening) by characterizing the efficacy and safety of these compounds, determining and understanding their mechanism of action (MOA) and predicting the likely human equivalent dose. One of our newest offering is helping clients assess the genotoxicity or mutagenicity of their test compounds.
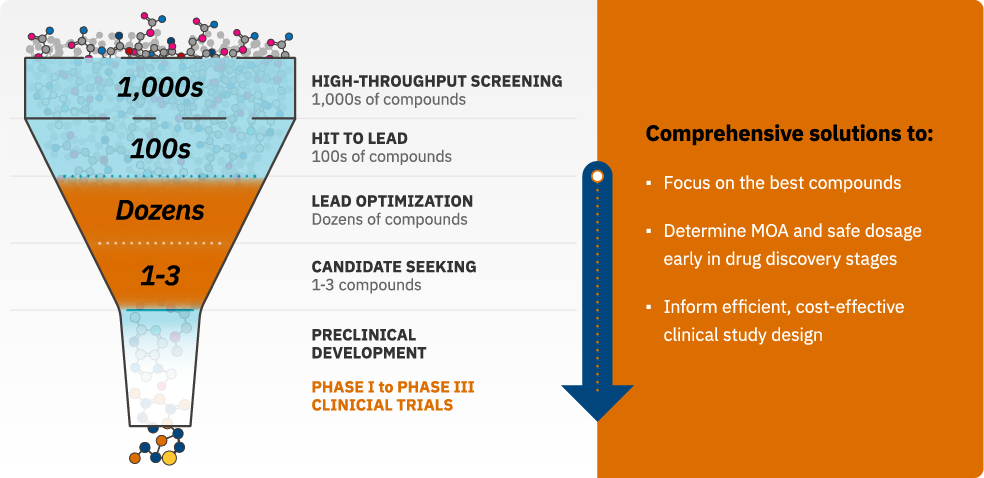
ScitoVation reduces time and costs to enable informed decisions.
Predicting equivalent human dose
For clinical testing, data from in vitro and animal studies coupled with PBPK modeling provides precise estimates of human equivalent dose for dosing regimens in early trials. With our PBPK tools, we help clients:
- Screen large library of compounds for safety
- Determining dose range for both in vitro and animal studies based on the structure of the compound
- Predicting human dose from animal or in vitro studies
- Drug-Drug Interaction (DDI): DDI risk simulation is the most popular application for PBPK modeling to avoid drug-drug interactions.
- Characterize intra- and inter-subject variability: Estimate variability across special populations (obese, elderly, infants or those affected by disease).
- Understand dose-effect relationships: Pharmacokinetic and pharmacodynamic modeling establishes the dose ranges between drug efficacy and safety to screen for drug acceptance and to prioritize next steps.
- Establish safety margins and efficacy characteristics: Margin of safety is the dose difference between the desired therapeutic effect and severe or life-threatening side effects.
Case Study: Understanding the mode of action of lead candidates for a compound targeting cancer
Case Study: Example of an in vitro assay followed by extrapolation to human for estrogenic compounds
