September 23, 2022
Development of an Internal Threshold of Toxicological Concern (iTTC)
Purpose
- With current regulatory mandates for reduction and elimination of animal experimentation, compounds developed for cosmetic ingredients and marketed in the European Union (EU) cannot be tested in animals. In response, research into a multitude of non-animal testing methods (in silico and in vitro) and safety evaluation techniques were pursued and contributed to the development of 21st century toxicology tools by the safety assessment community.
- The threshold of toxicological concern (TTC) is an important risk assessment tool that establishes acceptable low-level exposure values for risk-based prioritization and safety evaluations for chemicals with limited toxicological data. More importantly, the uncertainty at identifying an acceptable exposure can be reduced by assessing for the internal levels of chemicals in experimental animal and eventually in human. The internal metrics can be estimated by incorporating different biological and physiological factors such as chemical-specific information on the uptake, distribution, metabolism, and excretion of the chemical.
- The goal of this project was to use physiologically based pharmacokinetic (PBPK) modeling to estimate internal concentrations of pre-clinical toxicity studies so that internal threshold of toxicological concern (iTTCs) could be derived.
- The development of internal threshold of toxicological concern (iTTC) project was a part of the Cosmetics Europe Long Range Science Strategy (LRSS) 2016-2020 program.
Results
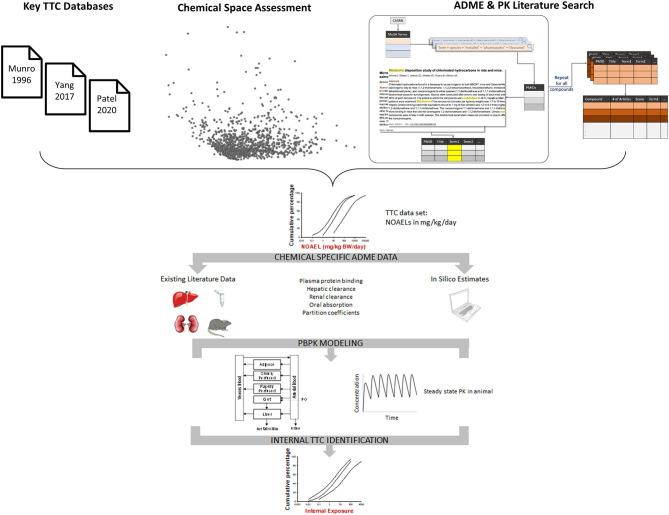
- A schematic of the iTTC Project from Ellison et al. (2021) shows the different steps of the project, beginning with combining the literature results, chemical space analysis, and TTC databases resulting in a list of ~350 chemicals for further review (now beyond 1400). Along with in silico calculated parameters, the existing literature data is being used to further refine the PBPK model while more data is being collected. After the modeling refinement is complete the resulting model will be used to evaluate data-poor chemicals and determine the iTTC.
- All the information collected was integrated into a computer (MySQL) database and web interface using R shiny package for simple access.
with empirically determined in vivo PoDs.
Client value
- Development of a generic PBPK model to predict iTTC chemical values.
- Incorporation of project information into a database for data sharing.
- Creation of web-based applications using the data.
- Visualization tools can be brought against the data.
