Our webinar speaker is Sudin Battacharya, PhD, Associate Professor in the Departments of Biomedical Engineering and Pharmacology & Toxicology at Michigan State University. Dr. Battacharya’s research sits at the crossroads of computation and biology, where he uses quantitative tools to study how signaling and transcriptional networks control cell fate and how environmental pollutants can disrupt these processes. In this talk, …
ScitoVation Awarded $2.04 Million SBIR Phase II Grant from NIEHS to Advance Predictive Toxicology Technology
DURHAM, NC – May 22, 2025: ScitoVation has been awarded a $2.04 million Phase II Small Business Innovation Research (SBIR) grant from the National Institute of Environmental Health Sciences (NIEHS), a part of the National Institutes of Health. The funding, distributed in $1 million increments over two years, will support the commercialization of ScitoVation’s DRIIVE platform (“Developmental and Reproductive In …
ScitoVation Announces Strategic Focus on Computational Toxicology: Laboratory Operations to Conclude March 31, 2025
February 18, 2025 – Durham, North Carolina: ScitoVation, a pioneer in New Approach Methodologies (NAMs), today announced a strategic decision to fully focus on advancing software tools and computational services to help clients assess the safety of compounds (in silico). By directing attention to its core strengths, ScitoVation aims to bolster its competitive edge and deliver even greater value to the …
Systems PBK/PD Modeling of 4-Hydroxyphenylpyruvate Dioxygenase Inhibition
Our webinar on PBPK Modeling features speakers Dr. Steven Webb and Dr. David Cowie from Syngenta. Together, they will share models to assess how Tyrosine aminotransferase (TAT) catalyzes the conversion of tyrosine to accumulation of hydroxyphenylpyruvic acid (HPPA) and how its activity varies among mammalian species. Species differences also exist in the ability to utilize alternative pathways for tyrosine catabolism …
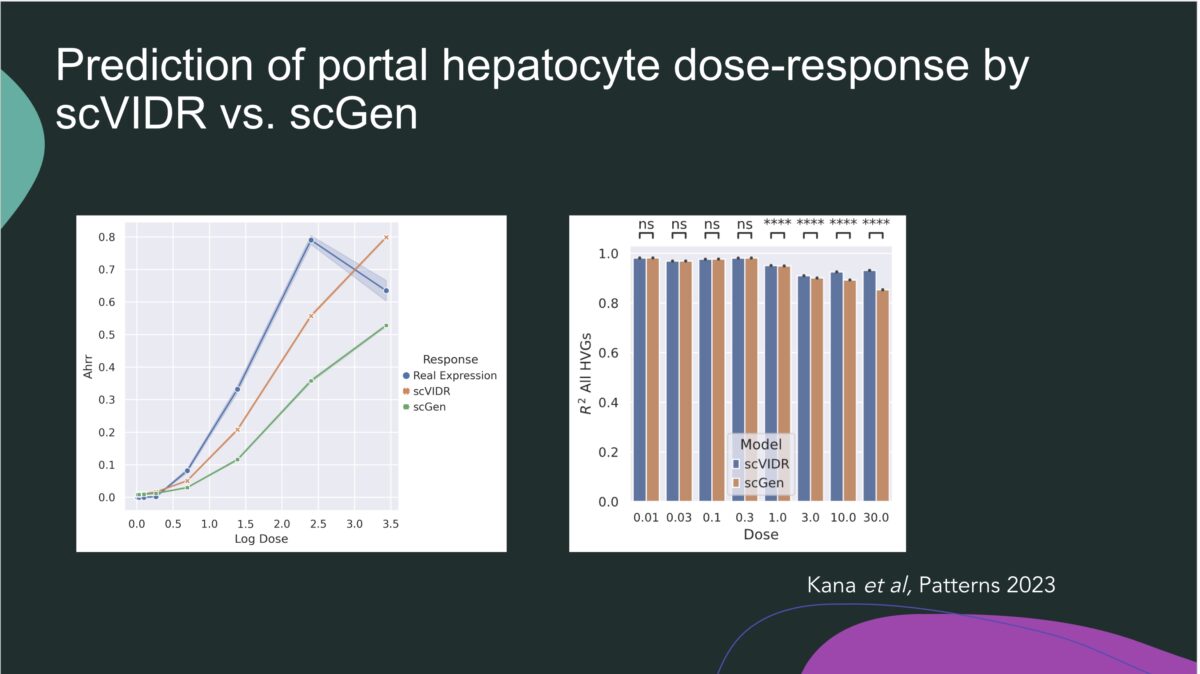
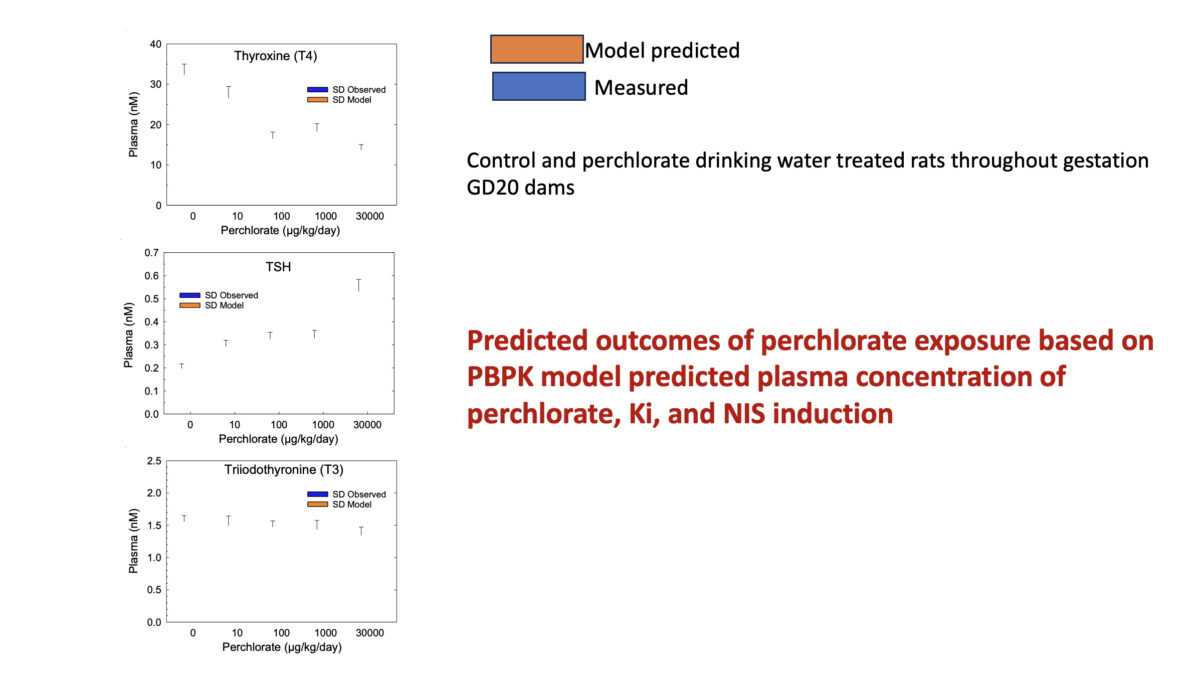
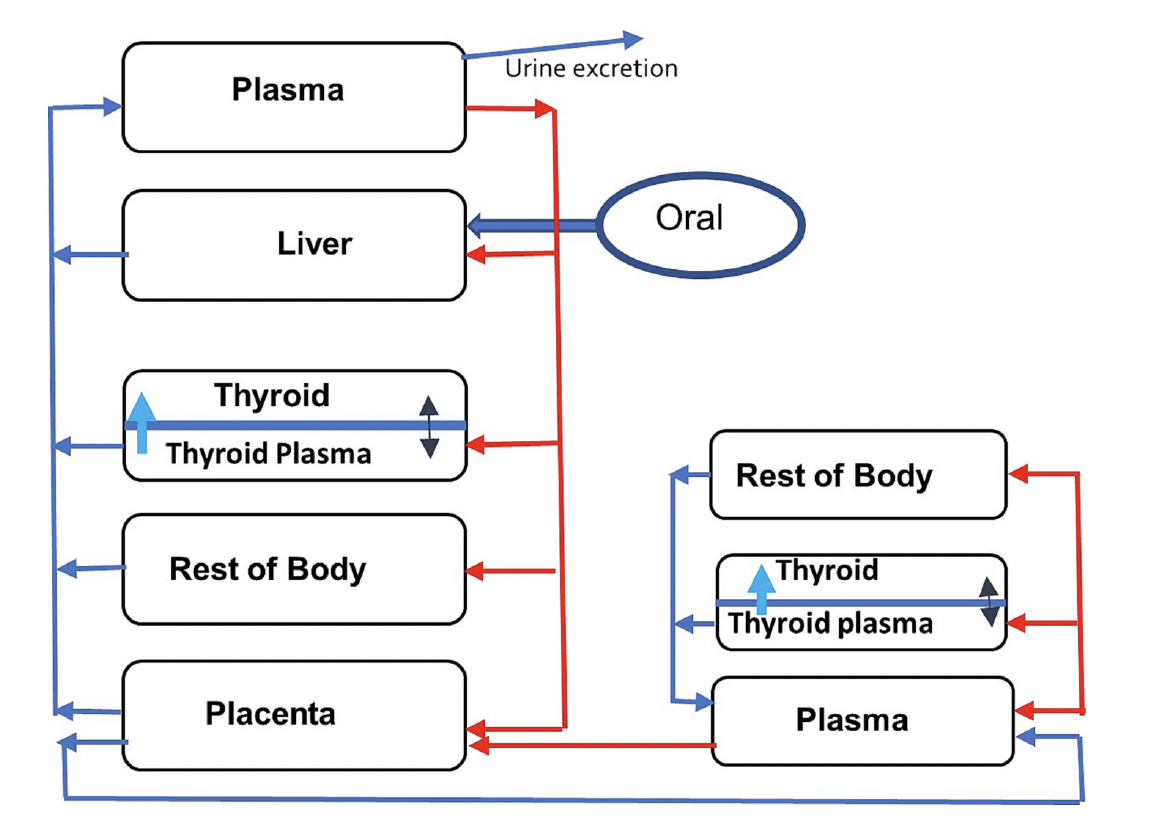
Towards Translating In Vitro Measures Of Thyroid Hormone System Disruption To In Vivo Responses In The Pregnant Rat Via A Biologically Based Dose Response (BBDR) Model
We are excited to present our first recorded installment for our 2024 webinar series. Jeff Fisher, PhD, ScitoVation’s Senior Science Fellow, will be speaking about thyroid disruption and IVIVE based on his latest publication. ScitoVation scientists continue advancing the benefits of IVIVE modeling. What you’ll learn: Thyroid modeling demonstrating IVIVE for a molecular initiating event determined in vitro How to …
Towards translating in vitro measures of thyroid hormone system disruption to in vivo responses in the pregnant rat via a biologically based dose response (BBDR) model
Jeffrey Fisher (a,*), Conrad Housand (b) David Mattie (c), Andy Nong (a), Marjory Moreau (a), Mary Gilbert (d) (a) ScitoVation, LLC, Research Triangle Park, NC 27713, USA (b) Magnolia Sciences, Winter Springs, FL, United States of America (c) AFRL/711 HPW/RHBAF, WPAFB, OH, United States of America (d) Office of Research and Development, Center for Public Health and Environmental Assessment, US …
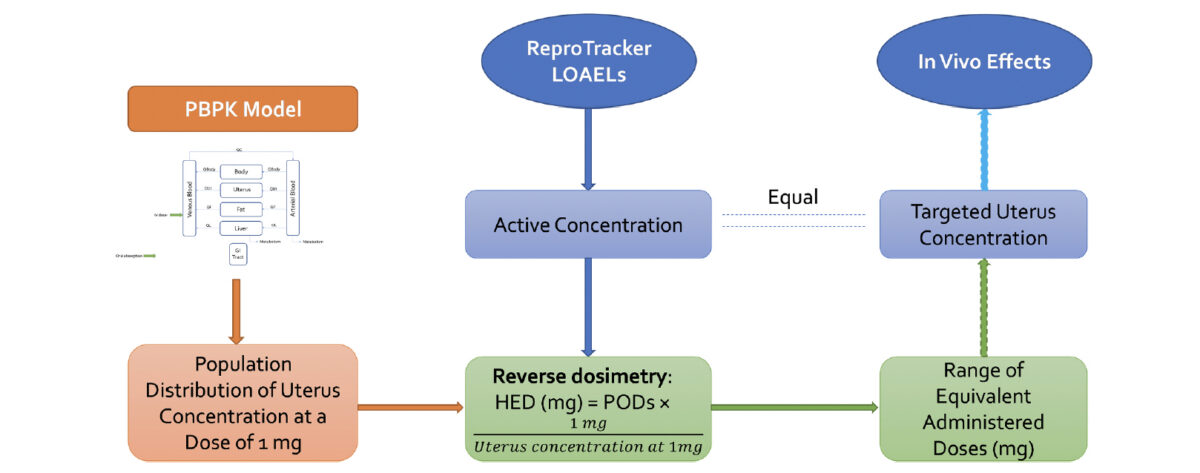
Animal-free assessment of developmental toxicity: Combining PBPK modeling with the ReproTracker assay
Marjory Moreau (a,*,1), Amer Jamalpoor (b,1,) John Carter Hall (a), Jeffrey Fisher (a), Sabine Hartvelt (b), Giel Hendriks (b), Andy Nong (a), Todor Antonijevic (a) (a) ScitoVation, LLC, Research Triangle Park, NC 27713, USA (b) Toxys, Leiden Bioscience Park, Oegstgeest, the Netherlands Abstract: in vitro screening platforms to assess teratogenic potential of compounds are emerging rapidly. ReproTracker is a …
Generic Pharmacokinetic Models for Mother-to-Offspring Transfer of Chemicals
Our speaker is Dustin Kapraun, PhD, physical scientist with the Center for Public Health and Environmental Assessment (CPHEA) in the Office of Research and Development (ORD) at the U.S. Environmental Protection Agency (EPA). In developing human health risk assessments, it is important to consider pregnant women, developing fetuses, and nursing children because chemical exposures experienced by these groups can lead …
ToxAIcology – the future of toxicology is AI
Artificial Intelligence (AI) is impacting all types of work, including toxicology. Dr Hartung will discuss how to move toxicology to a more holistic and integrated paradigm using AI and how AI plays a role in setting the direction of “Toxicology for the 21st Century 2.0” in future decades. What you’ll learn: Sources of Big Data for toxicology and various AI …
- Page 1 of 2
- 1
- 2