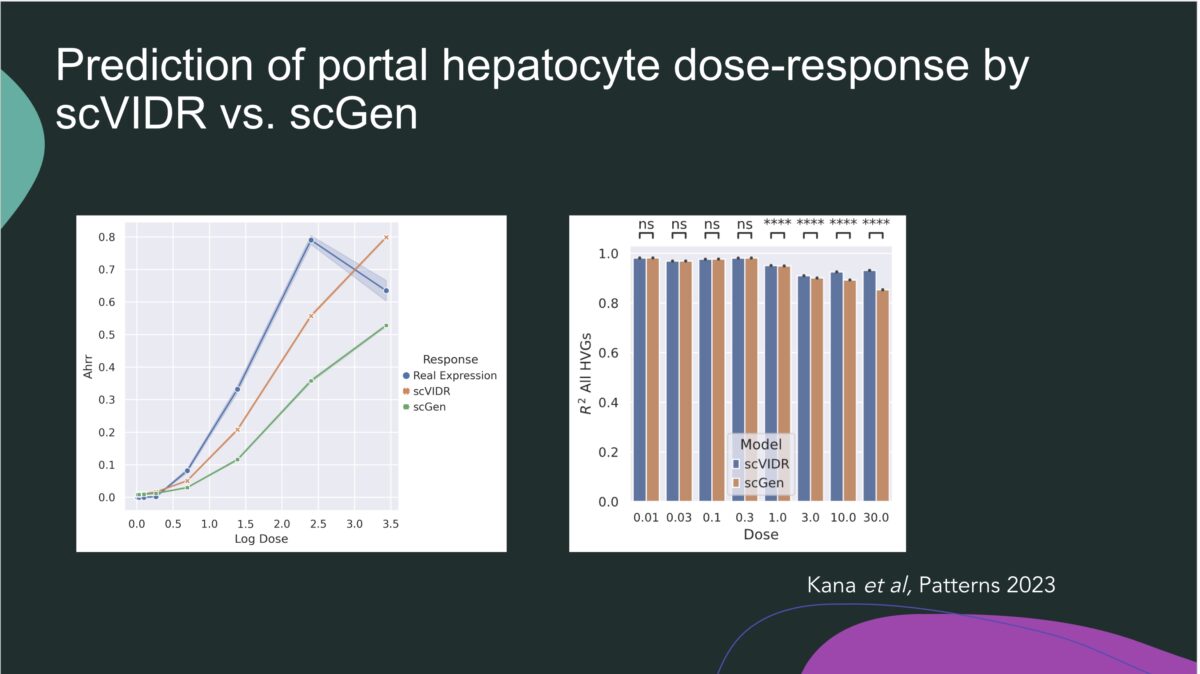
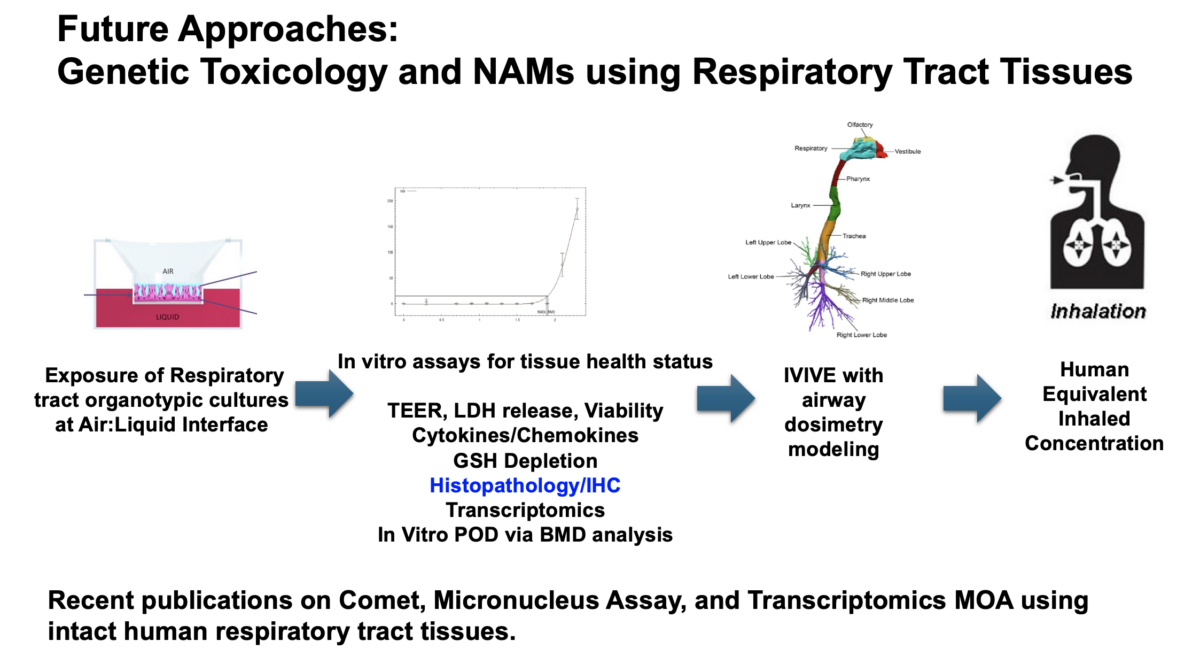
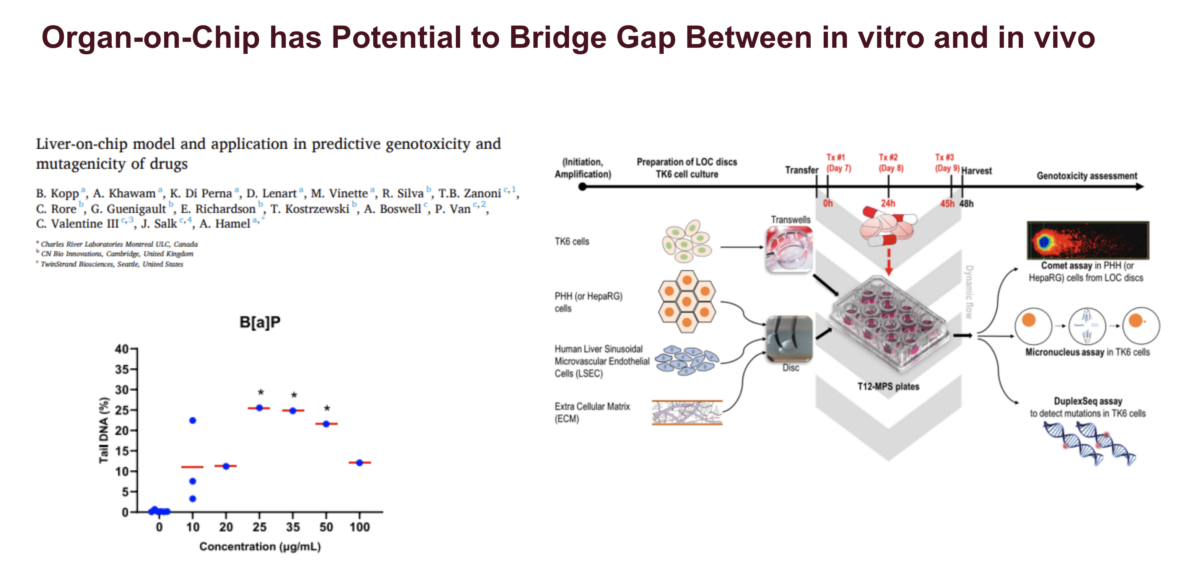
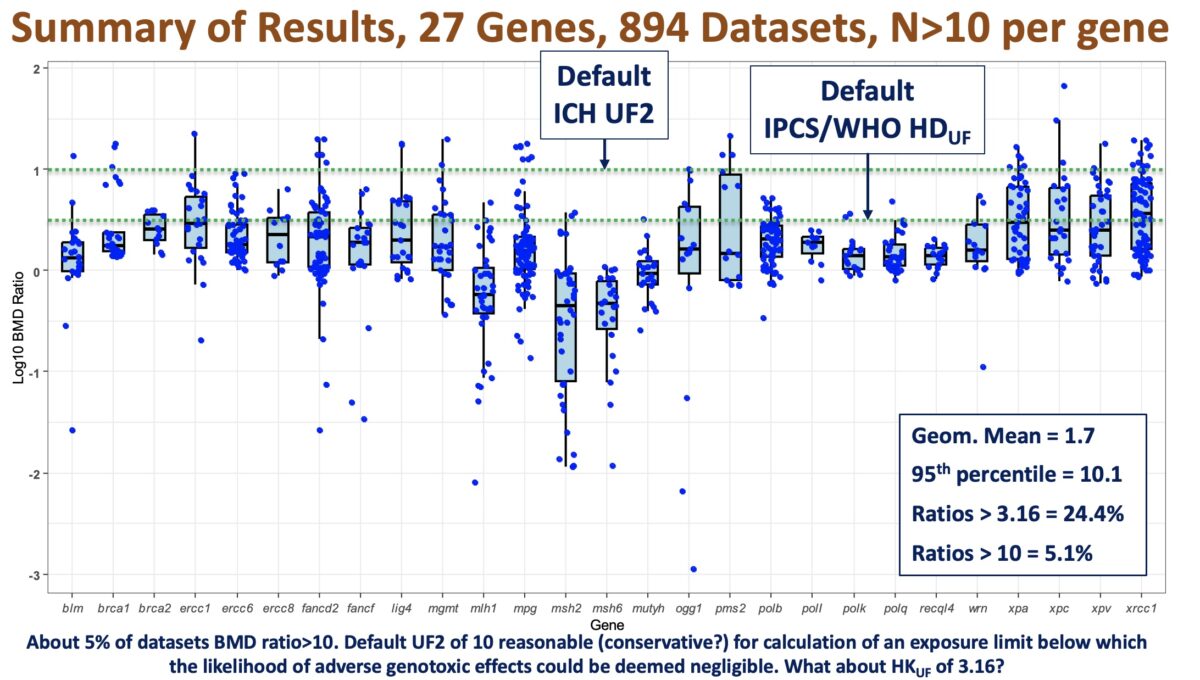
Our webinar speaker is Sudin Battacharya, PhD, Associate Professor in the Departments of Biomedical Engineering and Pharmacology & Toxicology at Michigan State University. Dr. Battacharya’s research sits at the crossroads of computation and biology, where he uses quantitative tools to study how signaling and transcriptional networks control cell fate and how environmental pollutants can disrupt these processes. In this talk, …
ScitoVation Awarded $2.04 Million SBIR Phase II Grant from NIEHS to Advance Predictive Toxicology Technology
DURHAM, NC – May 22, 2025: ScitoVation has been awarded a $2.04 million Phase II Small Business Innovation Research (SBIR) grant from the National Institute of Environmental Health Sciences (NIEHS), a part of the National Institutes of Health. The funding, distributed in $1 million increments over two years, will support the commercialization of ScitoVation’s DRIIVE platform (“Developmental and Reproductive In …
ScitoVation Announces Strategic Focus on Computational Toxicology: Laboratory Operations to Conclude March 31, 2025
February 18, 2025 – Durham, North Carolina: ScitoVation, a pioneer in New Approach Methodologies (NAMs), today announced a strategic decision to fully focus on advancing software tools and computational services to help clients assess the safety of compounds (in silico). By directing attention to its core strengths, ScitoVation aims to bolster its competitive edge and deliver even greater value to the …
Systems PBK/PD Modeling of 4-Hydroxyphenylpyruvate Dioxygenase Inhibition
Our webinar on PBPK Modeling features speakers Dr. Steven Webb and Dr. David Cowie from Syngenta. Together, they will share models to assess how Tyrosine aminotransferase (TAT) catalyzes the conversion of tyrosine to accumulation of hydroxyphenylpyruvic acid (HPPA) and how its activity varies among mammalian species. Species differences also exist in the ability to utilize alternative pathways for tyrosine catabolism …
Integrating computational tools and human cell-based NAMs for human-relevant risk assessments
Our first September 2024 webinar focuses on novel methods to assess relevant risk in humans with Les Recio, PhD, DABT, ATS, and Rasim Barutcu, PhD. We believe these approaches are a positive and proven way forward for expediting research and reducing the use of animals. While we do not offer a training course for integrating these tools, we do offer …
Quantitative Interpretation of in vitro Genotoxicity Data for Risk Assessment
If you attended /watched the recordings from the last two webinars, you might remember that Dr. Paul White and Dr. George Johnson highlighted the need to develop a conceptual framework to use genotoxicity data quantitatively for risk assessment. In this webinar, Marc Beal, PhD, a Research Scientist and Head of the Molecular and Applied Toxicology Section within the Food and …
Quantitative Interpretation of Genetic Toxicity Dose-response Data for Risk Assessment and Regulatory Decision-making: Opportunities, Challenges and Future Prospects
In continuing the momentum on the topic of quantitative interpretation of genotoxicity dose-response data for risk assessment, we have the pleasure of hosting Paul A. White, PhD as our next webinar speaker. Dr White is a research scientist at the Environmental Health Science and Research Bureau of Health Canada in Ottawa, Ontario, Canada. What you will learn: Assumptions underlying the …
Replacing Regulatory Default Assumptions of Linear Extrapolation of Risk with Benchmark Dose for Next Generation Genotoxicity Risk Assessments
Elevating NAMs in genotoxicity to reduce reliance on animal testing is a focus of our research efforts at ScitoVation. In addition to launching a 21st Century Genotoxicity program, we want to ensure you receive highly relevant information on the latest development in genotoxicity research. As such, we are pleased to host George Johnson PhD, from Swansea University. Dr Johnson is …
In vitro assessment of electronic nicotine delivery system (ENDS) aerosols
Dr Alexandra Noël speaks about using in vitro assays to assess the electronic nicotine delivery system aerosols. What you’ll learn: Overview of EVALI outbreak and harm caused. Known effects of e-cigarettes. How Air-liquid interface (ALI) physiologically relevant in vitro models can be used to assess the pulmonary effects associated with exposure to inhaled e-cig aerosols containing nicotine. Watch the Recording …