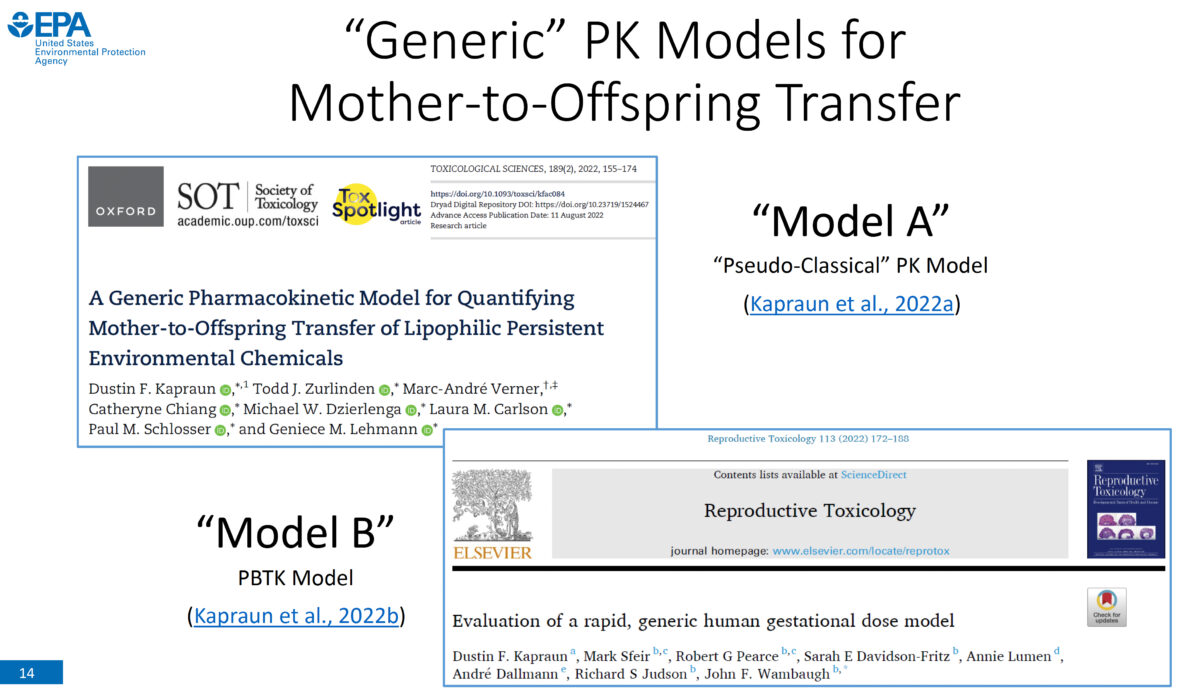
Our speaker is Dustin Kapraun, PhD, physical scientist with the Center for Public Health and Environmental Assessment (CPHEA) in the Office of Research and Development (ORD) at the U.S. Environmental Protection Agency (EPA). In developing human health risk assessments, it is important to consider pregnant women, developing fetuses, and nursing children because chemical exposures experienced by these groups can lead …
ToxAIcology – the future of toxicology is AI
Artificial Intelligence (AI) is impacting all types of work, including toxicology. Dr Hartung will discuss how to move toxicology to a more holistic and integrated paradigm using AI and how AI plays a role in setting the direction of “Toxicology for the 21st Century 2.0” in future decades. What you’ll learn: Sources of Big Data for toxicology and various AI …
Physiologically based pharmacokinetic modeling as a data analysis and study design tool – case studies in nonlinear pharmacokinetics
We just had a wonderful talk from Dr. Dan Hoer. Dr Hoer is a physical scientist with the US Environmental Protection Agency, Office of Pesticide Programs. His talk will focus on non-linear PBPK modeling, and the advantages offered by this approach. What you’ll learn: Important advantages of PBPK modeling over statistical methods that are often restricted to bimodal distinctions of …
ScitoVation Recipient of National Institute of Environmental Health Science Small Business Innovation Research Phase I Award of $269,977
DURHAM, N.C. (PRWEB) July 11, 2023: ScitoVation is the recipient of the National Institute of Environmental Health Science (NIEHS) Small Business Innovation Research (SBIR) Phase I award of $269,977. The SBIR program is a highly competitive program that encourages domestic small businesses to engage in Federal Research/Research and Development (R/R&D) with the potential for commercialization; enabling small businesses to explore …
(R)Evolution in Validation: Establishing Scientific Confidence in NAMs
Nicole Kleinstreuer, PhD, the director of the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) and the executive director of the congressionally mandated Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). spoke on July 18th, 2023. Dr. Kleinstreuer discussed a topic very important to our industry; a framework comprising five essential elements to establish scientific …
ScitoVation Recipient of National Institute of Environmental Health Science Small Business Innovation Research Phase II Award of $1.8 Million
DURHAM, N.C. (PRWEB) May 24, 2023 ScitoVation is the recipient of the National Institute of Environmental Health Science (NIEHS) Small Business Innovation Research (SBIR) Phase II award of $1.8 Million that is granted over two years in $900,000 increments. The SBIR program is a highly competitive program that encourages domestic small businesses to engage in Federal Research/Research and Development (R/R&D) …
A Source to Outcome Approach for Inhalation Risk Assessment: The Importance of Collaboration
Doug Wolf, DVM, PhD, discusses the creation and use of the Souce-to-Outcome framework, a new NAM incorporating a novel mathematical procedure developed to estimate the human equivalent concentration (HEC) for inhalation risk assessment based upon the relevant aerosol characterization, respiratory dosimetry modeling, and endpoints derived from an in vitro assay using human respiratory epithelial tissue. What you’ll learn: Introduction to …
NAM-Driven Computational Pipelines for Safety and Regulatory Framework
Andy Nong, PhD, ScitoVation’s Director of Computational Toxicology, is our guest speaker for this exciting webinar and new product announcement. Andy will share different computational pipelines that can complement a tier assessment approach for various forms of data such as in vitro screening tests or animal studies. What you’ll learn: How to integrate exposure and toxicity data in a quantitative …
Gene expression biomarkers as tools to interpret high-throughput transcriptomics data stream
We kicked 2023 off strong with our first webinar led by Dr. Chris Corton, Molecular Toxicologist in the Center for Computational Toxicology and Exposure at the U.S Environmental Protection Agency (EPA). Gene expression biomarkers are becoming increasingly important tools to assess mode of action (MOA), determine benchmark dose, and define point of departure for risk assessment. We welcomed Chris to …