Andy Nong, PhD, ScitoVation’s Director of Computational Toxicology, is our guest speaker for this exciting webinar and new product announcement. Andy will share different computational pipelines that can complement a tier assessment approach for various forms of data such as in vitro screening tests or animal studies. What you’ll learn: How to integrate exposure and toxicity data in a quantitative …
Gene expression biomarkers as tools to interpret high-throughput transcriptomics data stream
We kicked 2023 off strong with our first webinar led by Dr. Chris Corton, Molecular Toxicologist in the Center for Computational Toxicology and Exposure at the U.S Environmental Protection Agency (EPA). Gene expression biomarkers are becoming increasingly important tools to assess mode of action (MOA), determine benchmark dose, and define point of departure for risk assessment. We welcomed Chris to …
A revolution in mutagenicity testing: exploring the utility of error-corrected next-generation sequencing to quantify and characterize mutations
The second November installment of our webinar series is with Carole Yauk, PhD, University of Ottawa took place on November 29th at 11 ET. Dr. Yauk will explored the utility of error-corrected next-generation sequencing to quantify and characterize mutations. What you’ll learn: Introduction to error-corrected next-generation sequencing (ecNGS) and its advantages over conventional approaches in chemical mutagenicity assessment; Proof of …
New Approach Methodologies for Genetic Toxicity Assessment and Regulatory Evaluation of New and Existing Substances Description
The next installment of our webinar series is with Alexandra Long, PhD, from Health Canada. Alexandra will discuss NAMs as they relate to genetic toxicity. Dr. Long will provide an overview of an integrated, multi-endpoint, higher throughput, in vitro NAM platform, termed GeneTox21, led by Health Canada scientists, for the assessment of chemically induced genetic toxicity. What you’ll learn: The value …
Andy Nong Joins ScitoVation as Director of Computational Toxicology
DURHAM, N.C. (PRWEB)July 11, 2022 ScitoVation announced today the new Director of Computational Toxicology, Andy Nong PhD, formerly Health Canada’s Acting Manager of the Exposure and Biomonitoring Division and Principal Research Scientist for the Computational Toxicology Laboratory of the Environmental Health Sciences and Research Bureau. Dr Nong has been developing toxicokinetic and biological modeling research to advance alternative risk assessment …
ScitoVation Nabs Leslie Recio, PhD DABT, as Chief Scientific Officer
DURHAM, N.C. (PRWEB) APRIL 28, 2022 ScitoVation is pleased to announce Leslie Recio, PhD, DABT, as the new Chief Scientific Officer. Dr. Recio was the Chief Scientific Officer and Director of the Genetic and Molecular Toxicology group at ILS. Dr. Recio will help lead the team of Senior Scientists, drive priorities in New Approach Methods and help secure new business ventures. …
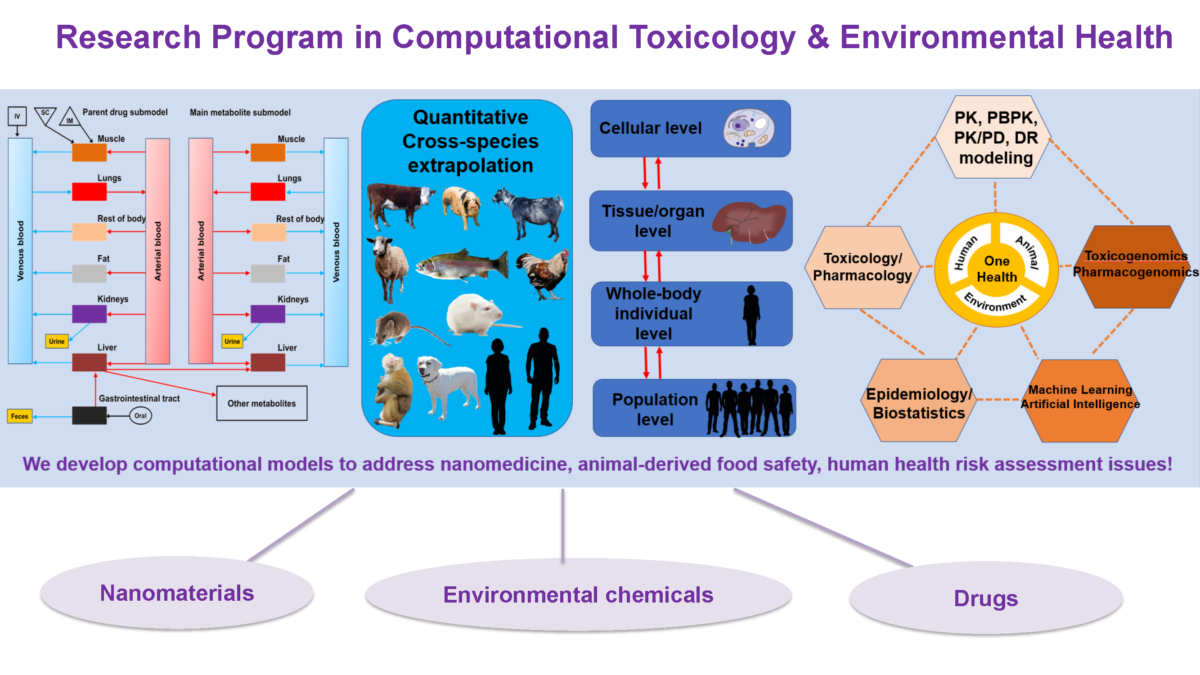
Applications of Physiologically Based Pharmacokinetic (PBPK) Modeling in Nanomedicine, Food Safety and Human Health Risk Assessment and Roles of Artificial Intelligence (AI) Approaches in these Areas
In this presentation, Dr. Zhoumeng Lin introduces how we develop physiologically based pharmacokinetic (PBPK) models of drugs, environmental chemicals and nanoparticles for applications in nanomedicine, food safety, and human health risk assessments. He presents his recent studies to illustrate each application. Dr. Zhoumeng Linalso introduces a new physiological parameter database for PBPK modeling in food-producing animals, including cattle, swine, sheep, …
What are NAMs?
by Sage Corzine If you got pumped up about NAMs in a toxicology webinar, tried to find more information on Google, and got redirected to the North American Menopause Society, you are not alone. NAMs or New Approach Methodologies are a vital and growing part of chemical hazard and risk assessment research. Here are a few fun facts …
The Frontier of Aerosol Safety Testing with In Vitro and in Silico Methods
by Scott Slattery In May and June of this year, the annual Webinar Series on Inhalation Toxicity Testing was co-hosted by The US EPA, the PETA Science Consortium International, Syngenta, and Unilever. The nine webinars presented over three days provided an excellent look at current progress in the area of non-animal inhalation toxicity testing approaches. The talks covered the breadth of the field, addressing in …