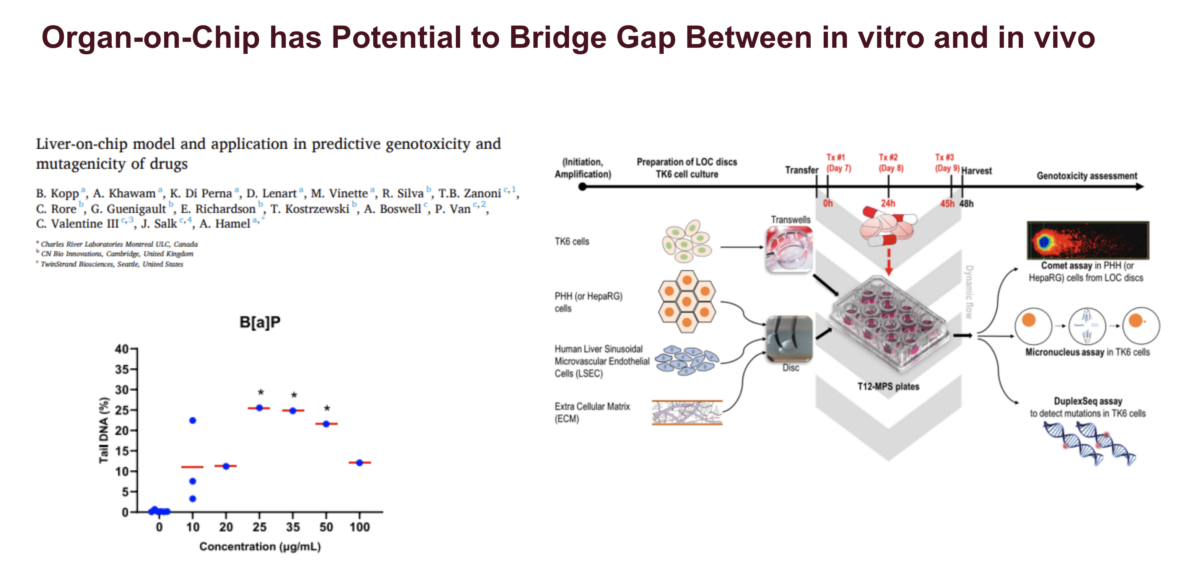
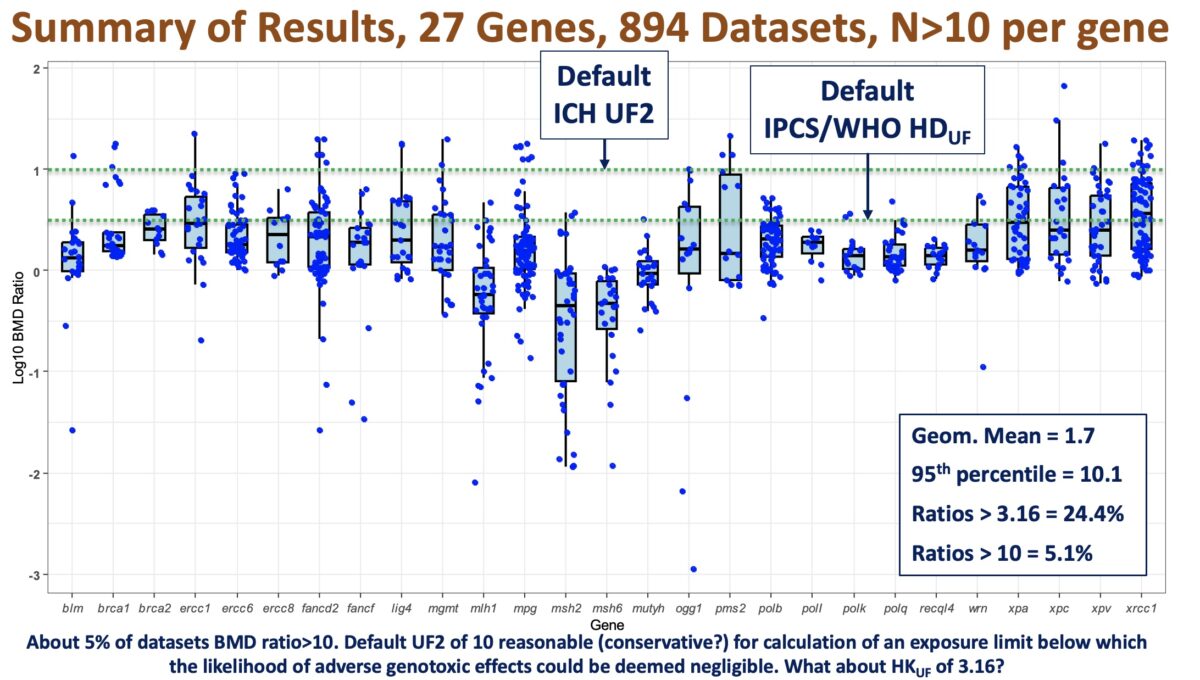
If you attended /watched the recordings from the last two webinars, you might remember that Dr. Paul White and Dr. George Johnson highlighted the need to develop a conceptual framework to use genotoxicity data quantitatively for risk assessment. In this webinar, Marc Beal, PhD, a Research Scientist and Head of the Molecular and Applied Toxicology Section within the Food and …
Quantitative Interpretation of Genetic Toxicity Dose-response Data for Risk Assessment and Regulatory Decision-making: Opportunities, Challenges and Future Prospects
In continuing the momentum on the topic of quantitative interpretation of genotoxicity dose-response data for risk assessment, we have the pleasure of hosting Paul A. White, PhD as our next webinar speaker. Dr White is a research scientist at the Environmental Health Science and Research Bureau of Health Canada in Ottawa, Ontario, Canada. What you will learn: Assumptions underlying the …