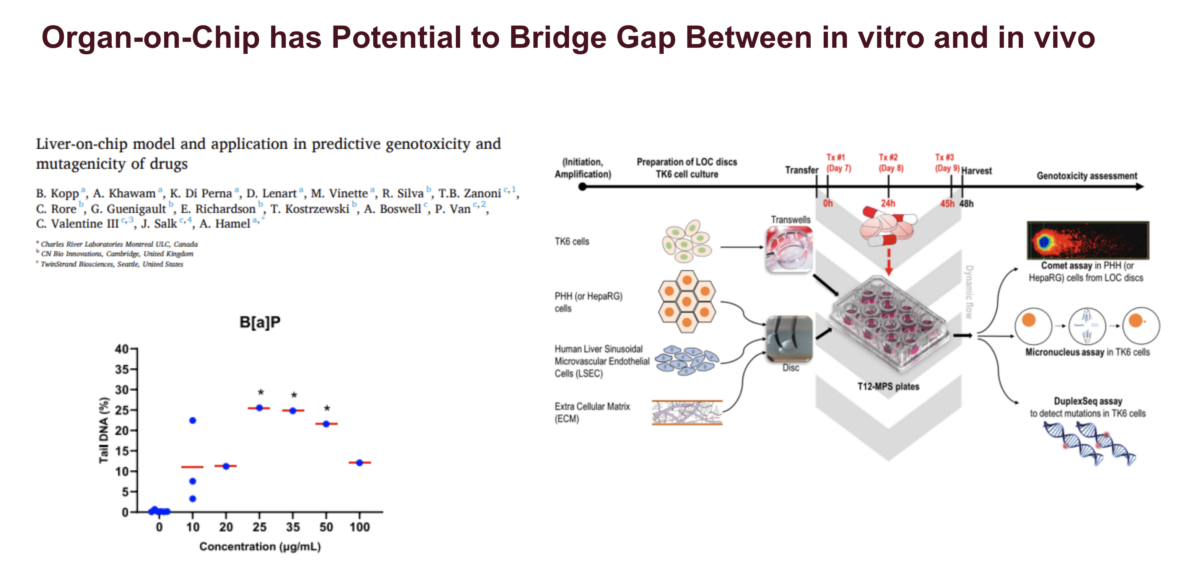
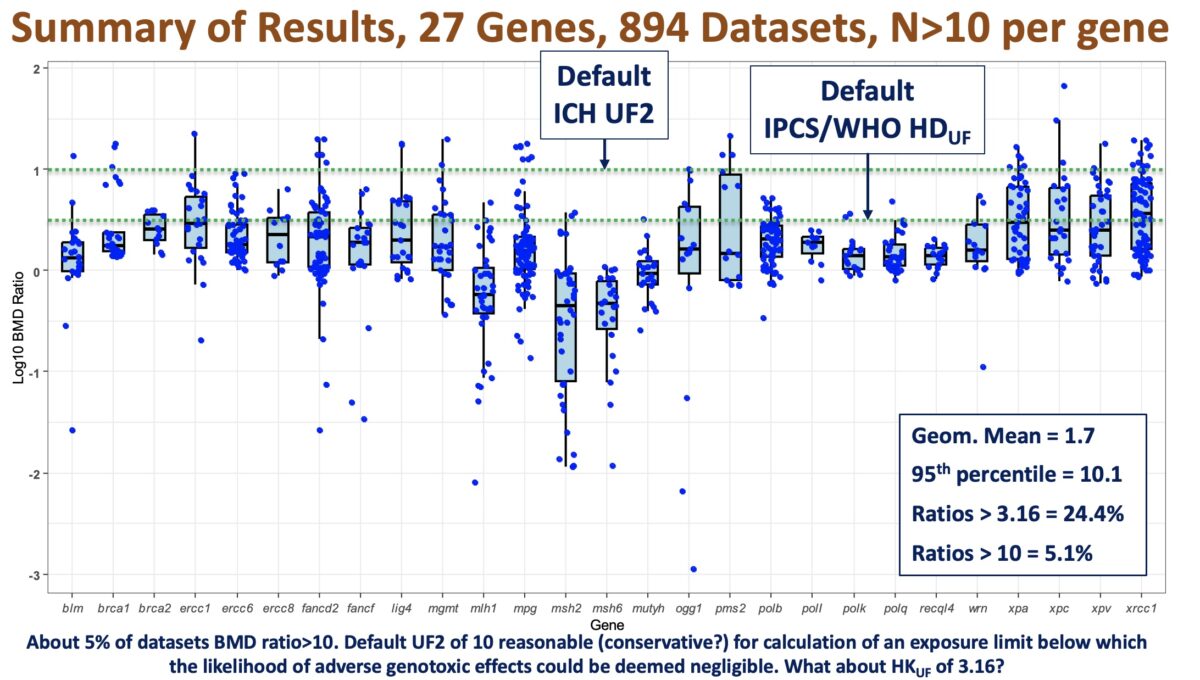
If you attended /watched the recordings from the last two webinars, you might remember that Dr. Paul White and Dr. George Johnson highlighted the need to develop a conceptual framework to use genotoxicity data quantitatively for risk assessment. In this webinar, Marc Beal, PhD, a Research Scientist and Head of the Molecular and Applied Toxicology Section within the Food and …
Quantitative Interpretation of Genetic Toxicity Dose-response Data for Risk Assessment and Regulatory Decision-making: Opportunities, Challenges and Future Prospects
In continuing the momentum on the topic of quantitative interpretation of genotoxicity dose-response data for risk assessment, we have the pleasure of hosting Paul A. White, PhD as our next webinar speaker. Dr White is a research scientist at the Environmental Health Science and Research Bureau of Health Canada in Ottawa, Ontario, Canada. What you will learn: Assumptions underlying the …
A revolution in mutagenicity testing: exploring the utility of error-corrected next-generation sequencing to quantify and characterize mutations
The second November installment of our webinar series is with Carole Yauk, PhD, University of Ottawa took place on November 29th at 11 ET. Dr. Yauk will explored the utility of error-corrected next-generation sequencing to quantify and characterize mutations. What you’ll learn: Introduction to error-corrected next-generation sequencing (ecNGS) and its advantages over conventional approaches in chemical mutagenicity assessment; Proof of …
New Approach Methodologies for Genetic Toxicity Assessment and Regulatory Evaluation of New and Existing Substances Description
The next installment of our webinar series is with Alexandra Long, PhD, from Health Canada. Alexandra will discuss NAMs as they relate to genetic toxicity. Dr. Long will provide an overview of an integrated, multi-endpoint, higher throughput, in vitro NAM platform, termed GeneTox21, led by Health Canada scientists, for the assessment of chemically induced genetic toxicity. What you’ll learn: The value …