In vitro to in vivo extrapolation (IVIVE)
integrating bioactivities into safety decision makingIVIVE is an integral component in new approach methodologies (NAMs)-based risk assessment paradigms for rapidly translating large-scale in vitro toxicity assay results in the context of in vivo exposure. There is additional value for data submission to regulatory bodies where the weight of evidence will be used. Regulators who may require the use of animal studies use dosimetry to answer questions such as:
"To what extent the doses in an in-life (animal) study correspond to what humans would be exposed to under normal use or based on extraordinary circumstances such as occupational exposure?"
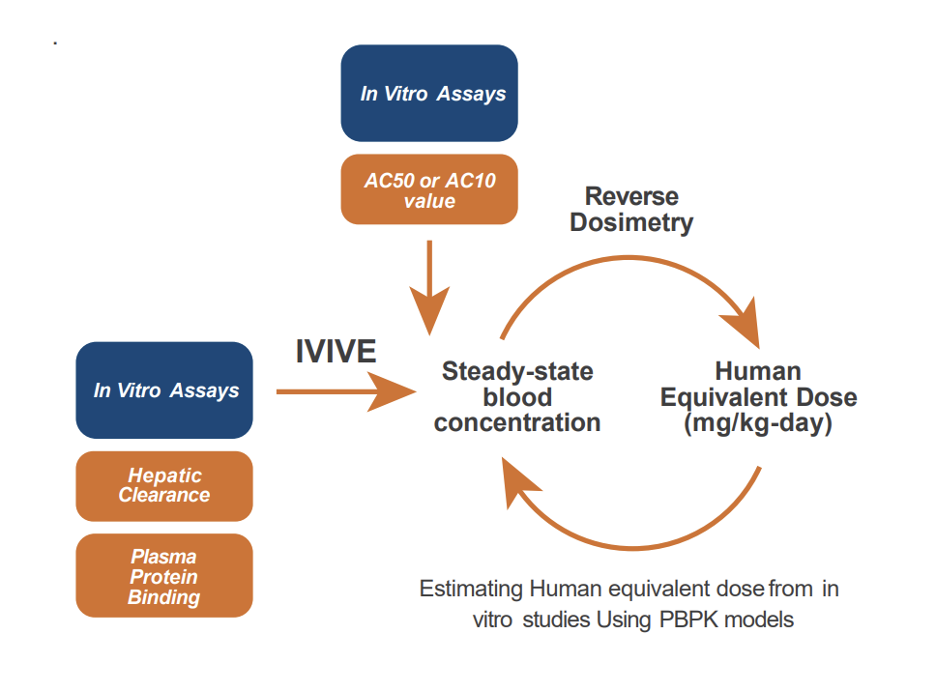
Models of dosimetry such as high-throughput IVIVE can be used to estimate the relationship between an in vitro concentration of interest—such as a benchmark dose estimate—and the whole-animal or human exposure that would lead to the equivalent target tissue concentration. An example of recently published HT-IVIVE work is the development and validation of a fit-for-purpose assay that predicts tissue-specific responses to estrogens (i.e., breast vs. uterine differences) and would also provide dose-response information sufficient to derive points of departure (PODs) for human risk assessment. Quantitative in vitro to in vivo extrapolation (Q-IVIVE) was performed to convert the in vitro concentrations from the assay to external human equivalent doses (HEDs). Its use is very valuable for prioritizing the health risk of chemicals.
For more complex scenarios, we use pharmacokinetic modeling or physiologically based pharmacokinetic (PBPK) modeling tools to convert chemical concentrations active in experimental in vitro systems equivalent exposure levels in vivo via reverse dosimetry.
ScitoVation provides pharmacokinetic modeling at various levels of complexity. Our PBPK/IVIVE modeling capabilities are tunable to meet your needs.
Case Study: Example Of A Custom Assay Followed By IVIVE To Prioritize Compounds
Case Study: Translating In Vitro Inhalation Study To Human Equivalence
Case Study: Use of PBPK Modeling to Support Risk Assessment for Susceptible Populations
