Client problem
Pyrethroids are among the most commonly used insecticides. Concerns for potentially higher sensitivity to pyrethroid exposure in children arose from previous studies showing that rat juveniles were more sensitive than adults to pyrethroids. In response to US Environmental Protection Agency (USEPA) questions about the relevance of the high dose rat data, the Council for the Advancement of Pyrethroid Human Risk Assessment (CAPRHA) was formed to investigate whether children are more sensitive to pyrethroid neurotoxicity than adults.
ScitoVation Solution
Under the new toxicity testing paradigm that is largely based on in vitro and in silico approaches, alternative strategies are needed to address risk to potentially human sensitive populations like children in chemical risk assessment.
To address the concern for age-related sensitivity to pyrethroids, life-stage physiologically based pharmacokinetic (PBPK) modeling supported by in vitro to in vivo extrapolation (IVIVE) was conducted to predict age dependent changes in target tissue exposure to pyrethroids.
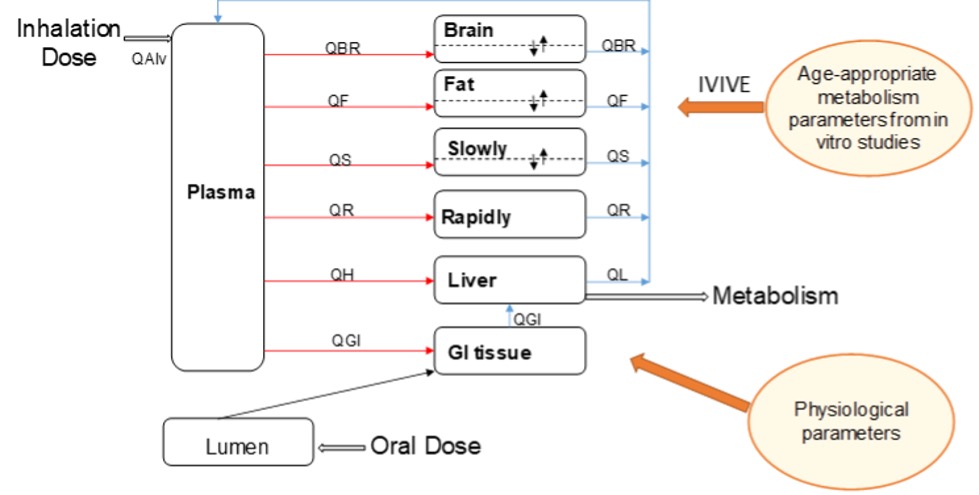
Figure 1. Structure of a PBPK model using IVIVE for metabolism parameterization.
For the purpose of developing a safe dose level to the human population, including sensitive subgroups like children, regulators apply safety factors to account for limitations in the data used and variability and uncertainty in the differences between animals and humans. The purpose of this early age PBPK model is to calculate a chemical specific adjustment factor (CSAF) to address age-related pharmacokinetic (PK) differences for pyrethroids in humans.
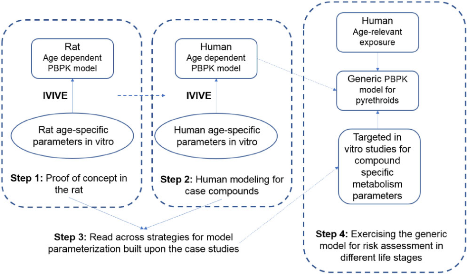
Figure 2. The approach building a generic physiologically based pharmacokinetic (PBPK) model for pyrethroids.
We showed that our IVIVE approach coupled to PBPK modeling accurately captures pharmacokinetic effects in rats. We built on this success by developing the human life stage PBPK model that was used to predict brain maximal concentrations (Cmax) in adults and children. This knowledge enabled the calculation of a CSAF for humans, to replace the default 3X Food Quality Safety Act (FQPA) safety factor used in quantitative risk assessment to protect children.
Traditionally, exercising the PBPK model to obtain point of departure (POD) requires technical expertise in running the software in which modeling was performed. We have developed an interactive modeling platform called Population Lifecourse Exposure-To-Health-Effects Model (PLETHEM) to make the PBPK modeling more accessible to risk assessors and chemical safety practitioners.
Results from our work
Our results show that, based on the use of the Life Stage PBPK models with different pyrethroids, CSAF values are essentially equal to or less than 1, resulting in a CSAF for age related PK differences of 1. For risk assessment purposes, this indicates that no additional adjustment factor is necessary to account for age-related PK differences for these pyrethroids.
Another application of the PBPK model is the calculation of PODs based on specific scenarios in a risk assessment context.
The life stage modeling framework supported by IVIVE parameterization can be readily applicable to other chemicals with potential concerns for early life sensitivity.
