Purpose
- Risk assessment is the characterization of the potential adverse effects in humans to exposures of environmental hazards. Traditional risk assessment methods include determining a point of departure (POD) from animal toxicity studies and calculating a human reference dose using uncertainty factors (UF) to account for data limitations and variability and uncertainty resulting from differences between and within test animals and humans.
- In this project, to address the concern for age-related or disease-related sensitivity to chemicals we showed how a physiologically based pharmacokinetic (PBPK) model coupled with in vitro to in vivo extrapolation (IVIVE) can be used to predict internal concentrations of chemical X in different populations (adults, children and renally impaired population). We used a “parallelogram approach” (Figure 1) to describe the development, calibration, and validation of the model in rat and human.
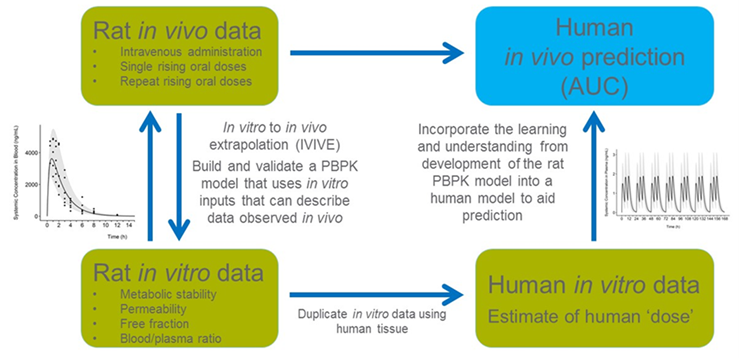
FIGURE 1 Parallelogram approach to predict human internal dosimetry.
The approach of predicting in vivo metabolic clearance based on in vitro data using IVIVE has gained strong support in recent years, especially with the publication of the recent guidance document from the Organization of Economic Cooperation and Development (OECD)1. As risk assessment is shifting from animal testing to new approaches based on in vitro and in silico methods, alternative strategies are needed to accurately integrate and use this non-animal data.
- Both in vitro metabolism parameters and in vivo kinetic data were developed for chemical X and its metabolite in rat. The model was used to recapitulate these in vivo pharmacokinetic results.
- The IVIVE approach was then similarly applied to the human model development. The blood area under the curve (AUC) was used as the dose metric to compare between species. The model allows a quantitative determination of interspecies extrapolation and human intraspecies variability between subpopulations.
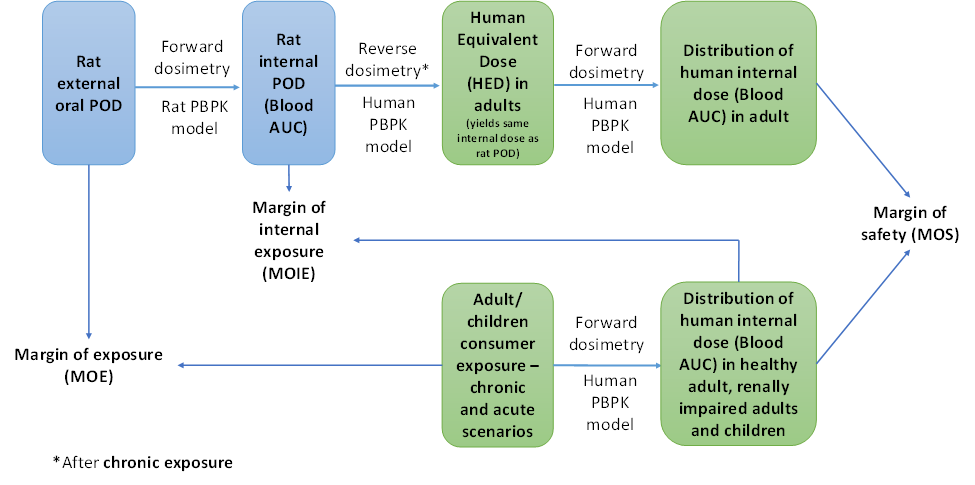
FIGURE 2 Calculation of MOIE and MOS.
From the internal exposure model predictions, it was possible to calculate the margin of internal exposure (MOIE) and the margin of safety. The ability to rely on a measure of internal rather than external exposure reduces the uncertainty in the risk assessment by incorporating chemical-specific information on the uptake, distribution, metabolism, and excretion of the chemical in both the experimental animal and the human.
Results
- The PBPK model was used to predict human internal dose metrics of various age groups (children and adult) including renally impaired adults. The resulting internal dose metrics were compared against equivalent rat (oral) POD metrics. The aggregate exposure values were then measured in terms of a MOIE and MOS.
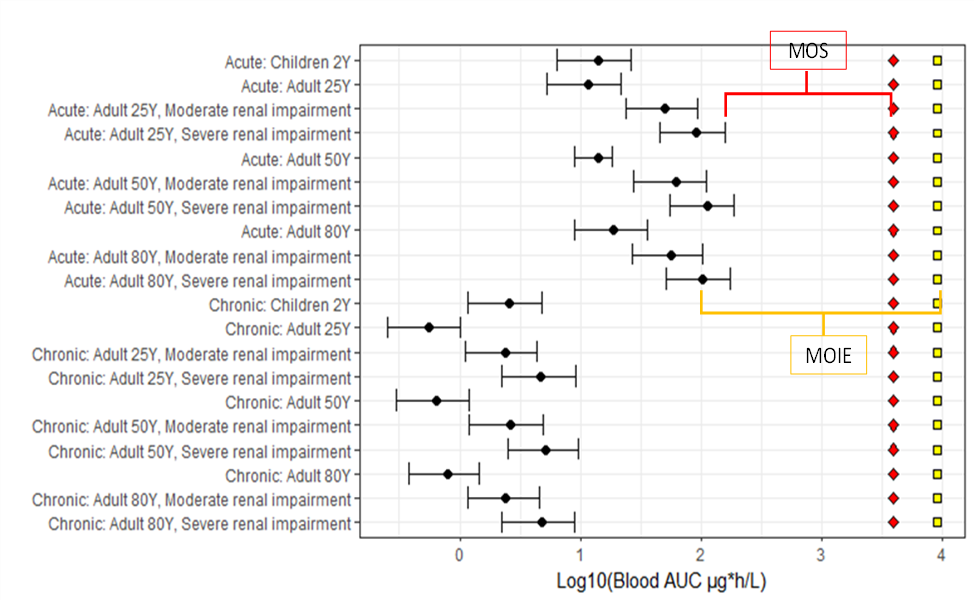
FIGURE 3 Margin of internal exposure (MOIE) and margin of safety (MOS). Black point represents the mean AUC24, and the lower and higher bars represents the 1st and 99th percentiles. The red diamond is the 1st percentile AUC24 at the human equivalent POD and the yellow square is the mean AUC at the POD.
Client Value
- We are dedicated to work in partnership with our client to support decision making processes at various stages along the life cycle of a chemical or drug from research through development. We build knowledge to improve the understanding of a chemical and we deliver information that will help the client in decision making or regulatory submission.
- In this project, we have predicted exposure from one species (rat) to another (human). In this case, you have a rat POD for a given concentration in blood and you would like to estimate the dose of exposure giving the same blood concentration in human.
- We have captured human variability by including monte Carlo analysis in the PBPK model.
- We have estimated a response from varying exposure conditions and across life-stages or vulnerable populations, such as children, or renally impaired populations.
