Respiratory Toxicity
Cell-Based Assays Integrated with In Vitro to In Vivo Extrapolation Modeling for Risk Assessments
Human based New Alternative Approach Methodologies (NAM) to risk assessment eliminate uncertainties and known differences associated with extrapolation from rodent studies to human outcomes. ScitoVation respiratory toxicology research uses NAM based dose−response data integrated with Physiologically Based Pharmacokinetic (PBPK) and In Vitro to In Vivo Extrapolation (IVIVE) modeling as an approach to determine human equivalent exposures (HEC) and risk assessment from exposure to potential inhalation hazards.
Air:Lung Interface Exposure and Organotypic cell culture models
Inhalation is an important route of exposure to gases, volatile compounds, and particles that can differentially affect the respiratory tract from nasal and bronchiolar airways to the alveoli. The respiratory tract is exposed to ambient air and is a first point of contact within the body.

Air:Lung Interface (ALI) systems are used to expose lung cells and lung organotypic cultures to vapors, gases, and aerosols mimicking in vivo exposure occurs that occur at the interface of the atmosphere and respiratory tract. ScitoVation has two modes of ALI exposure using a VitroCell® 12/12 exposure system, enabling simultaneous and continuous exposure of 12 ALI cell culture samples to four different concentrations of a gas or vapor (usually three treatment concentrations and a clean air control). Secondly, ScitoVation has recently established the VitroCell® Cloud Alpha 6 to enable direct exposure of mammalian cells or tissue at the ALI to aerosols, particles, and semi-volatile compounds. The Cloud system includes a microbalance enabling quantification of deposited dose. These two complimentary exposure systems can be used to meet ScitoVation client needs for required testing of potential inhalation toxicity.
NAMs in Respiratory Toxicology
The reduction in the use of animals and shift towards human cell-based NAM for respiratory toxicology requires the use of respiratory tissue dosimetry and human relevant tissue models to estimate HEC and safety margin of exposure for inhalation risk assessments. ALI models to assess respiratory toxicity include human bronchial epithelial cell culture models from primary cells or alveolar organoids derived from human pluripotent stem cells differentiated, and ex-vivo precision-cut lung slices (hPCLS) that contain all lung cell types present in the tissue at the time and retains the native architecture of the lung.
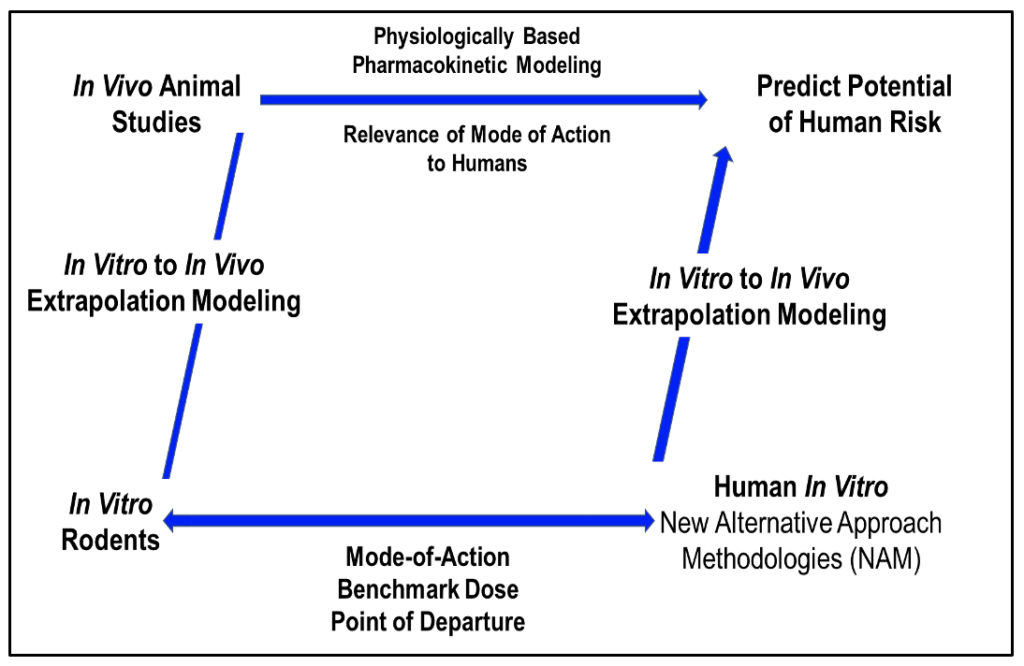 ScitoVation scientists have pioneered the integration of apical measures of tissue toxicity and Mode-of-Action (MOA) based genomic pathways analysis with PBPK modeling for use in risk assessments. As toxicology transitions away from reliance on animals, ScitoVation scientist are focused on conducting dose-response studies in human-relevant tissue models to calculate Benchmark Dose (BMD) and Point of Departure (PoD) from apical and genomic endpoints and using IVIVE modeling to estimate a HEC for use in risk assessment.
ScitoVation scientists have pioneered the integration of apical measures of tissue toxicity and Mode-of-Action (MOA) based genomic pathways analysis with PBPK modeling for use in risk assessments. As toxicology transitions away from reliance on animals, ScitoVation scientist are focused on conducting dose-response studies in human-relevant tissue models to calculate Benchmark Dose (BMD) and Point of Departure (PoD) from apical and genomic endpoints and using IVIVE modeling to estimate a HEC for use in risk assessment.
Case Studies
ScitoVation scientists are using a NAM based parallelogram approach to risk assessments implementing cell-based BMD and POD from apical and genomic endpoints with IVIVE modeling to estimate potential in vivo tissue levels and predict potential outcomes in exposed humans. IVIVE derived estimation of human equivalent concentration (HEC) from in vitro studies is needed to assess relevance of in vitro findings to potential in vivo outcomes.
- ScitoVation scientist have used in vivo airway dosimetry models for 1,3-dichloropropene vapor exposures to human respiratory track primary cells at air:lung interface to predict in vivo external exposure scenarios that would produce toxic local tissue concentrations as determined by in vitro experiments. Although predicted PoD concentrations were slightly higher than PoDs determined by in vivo sub chronic studies, these studies are highly encouraging and model refinement is ongoing to increase accuracy of IVIVE modeling (Moreau et al., 2022).
- Syngenta scientists used a NAM based approach to conduct a risk assessment for exposure to products containing the broad-spectrum fungicide chlorothalonil (Ramanarayanan et al., 2022). The HECs for inhalation exposure to chlorothalonil using this NAM based approach resulted in margins of exposure ranging from 230X to 70,000X that of potential human exposures.
These case studies present a roadmap for using NAMs integrated with IVIVE and are a significant step in modernizing toxicology risk assessments by relying on human based test systems rather than rodents.
Moreau et al., 2022 NAM-based prediction of point-of-contact toxicity in the lung: A case example with 1,3-dichloropropene. Toxicology (in press)
Ramanarayanan et al., (2022) Application of a new approach method (NAM) for inhalation risk assessment. Regul Toxicol Pharmacol. 133:105216
