Kinetic Proliferation Assay
The Kinetic Proliferation Assay is used to understand proliferation rate of a cell line with or without treatment by counting the number of cells in samples at different timepoints after seeding and treatment. The assay determines compound effects by measuring basal and treated proliferation rates of a cell line. For a client, we used the proliferation assay to show that a cancer cell line treated with their compounds had lower proliferation rates compared to cells with a vehicle control.
This assay can be run as a fixed-and-stained-cell assay with a variety of cell types. Alternatively, an initial investment in the generation of stable cell lines with GFP nuclear tags allows for a more efficient live-cell assay. This base assay is potentially multiplexable with additional fluorescent probes and readouts, such as cytotoxicity, nuclear morphology, or immunocytochemistry.
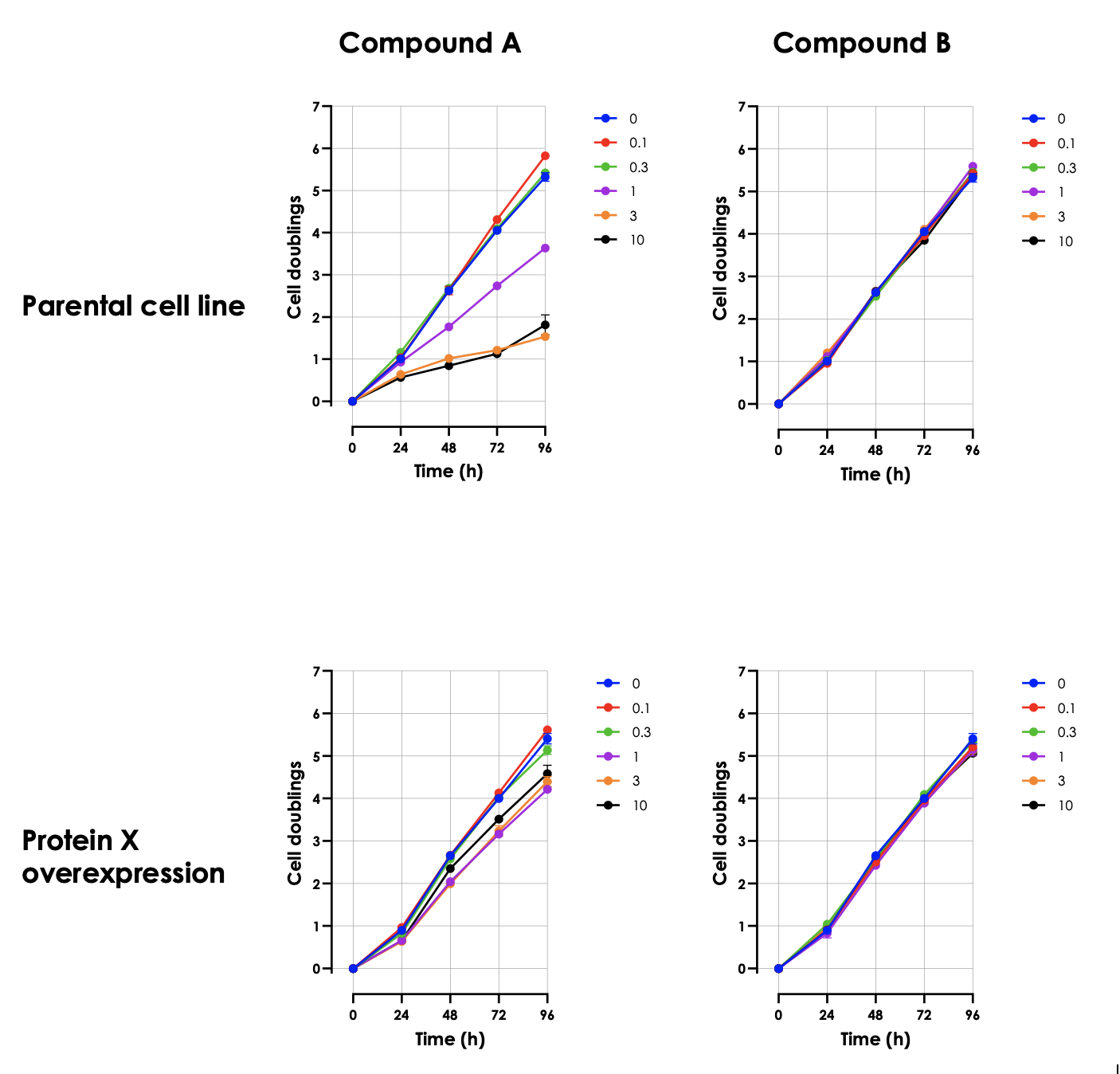
Kinetic Proliferation Assay example. A parental cell line (top plots) or a line derived from the parental line by overexpression of protein X (bottom plots) were treated with active Compound A (left plots) or a structurally related but inactive Compound B (right plots) at the indicated concentrations. Cells per well were counted by high content imaging at 24-h intervals from 0 to 96 h after treatment. Counts were converted to doublings relative to time 0 for plotting. Points are means and error bars are standard deviations of triplicate wells. Compound A reduced the proliferation rate of the parental cell line in a dose-dependent manner, whereas Compound B had no effect on the parental line. Overexpression of Protein X suppressed the anti-proliferative effect of Compound A.
