Genotoxicity
Non-Animal Human-Relevant Genotoxicity Assessments for Lead Candidate Selection
Knowledge regarding the potential of pharmaceuticals, food additives or chemicals to damage the human genome and cause mutations remains critical to public health. Mutations are key events in the induction of cancer, birth defects, and neurological disease. The regulatory genetic toxicology test battery required by the US Food and Drug Administration (FDA) for Investigational New Drug applications to initiate clinical trials, and by the US Environmental Agency (EPA) for registration of pesticides, includes approved in vitro tests for assessing genotoxicity and mutagenicity. A positive response in this regulatory test battery can eliminate drug candidates from further development or require further in vivo testing to demonstrate safety. These in vivo tests add significant time to development and can be costly.
ScitoVation’s genotoxicity program is aimed at eliminating potential genotoxicity hazards early. This saves time and resources by reducing positive outcomes in the regulatory genetic toxicology test battery that can stop further development. Current genetic toxicology testing is plagued with long backlogs for study starts, outdated technologies focused on rodent cell lines, and genotoxicity assessment using high dose animal testing (up to 2000 mg/kg) with significant risk assessment assumptions used for extrapolation of those data to humans.
What we offer:
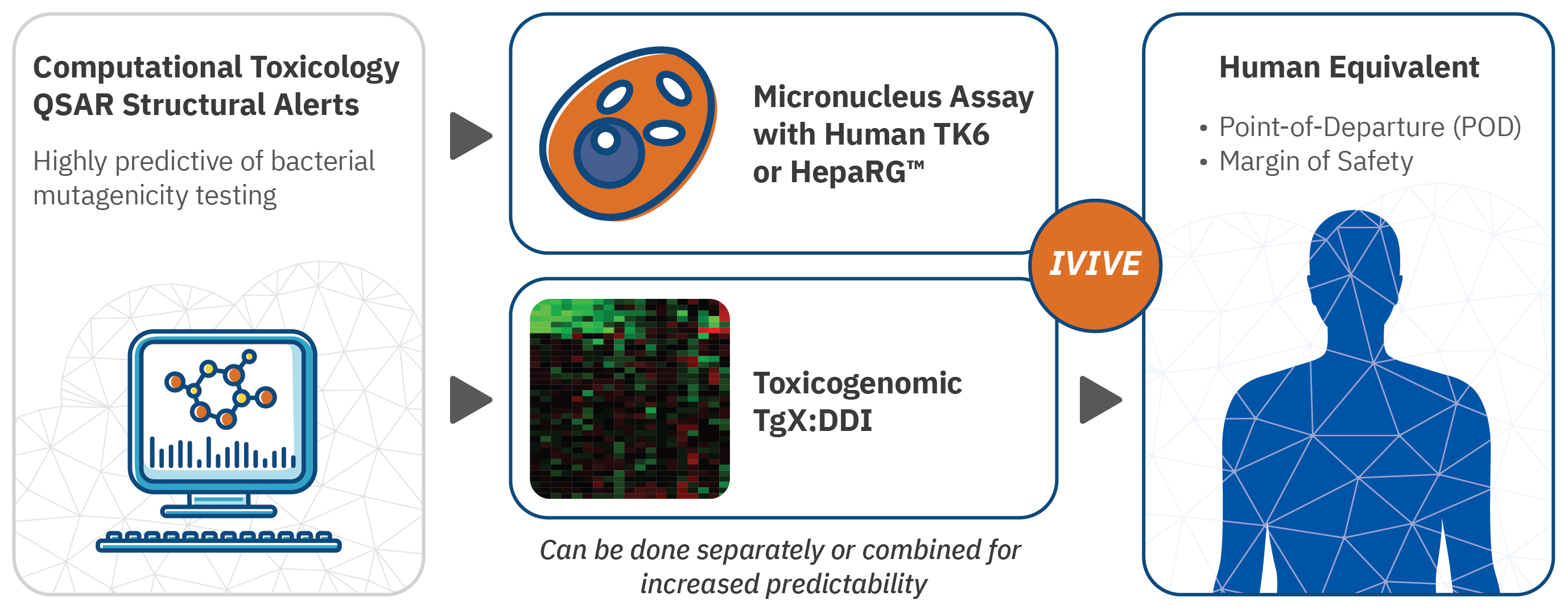
- Computational tools based on compound structure for screening early candidates that are highly predictive of bacterial mutagenicity testing.
- For lead candidates, the approved in vitro micronucleus assay using human TK6 or HepaRG cells.
- Our depth of expertise in toxicogenomics, enables us to offer the TgX DDI biomarker, which is currently undergoing FDA validation as biomarker predictive of mammalian genotoxicity. The advantage of using TgX DDI is that it provides a point of departure that can be used to translate the in vitro results to human equivalent dose.
Each stage can be offered separately, depending on the needs of the client.
Program Benefits:
- Limited backlog.
- Human-based non-animal testing.
- Access to staff with recognized expertise in genotoxicity and computational toxicology who can help address challenges.
- Nimble organization that understands the needs of small biotech companies.
Next Generation Genotoxicity Assessments
Computational Tool for Testing Mutagenicity (Ames Test)
We use quantitative structural-activity relationship (QSAR) tools that are highly predictive of bacterial mutagenicity test (Ames Test). The use of QSAR offers an efficient means from both cost and timing perspective to triage candidates and focus additional in vitro testing on more promising candidates.
Micronucleus Assay
We use P53 proficient human TK6 cells to screen for the flow cytometry based in vitro micronucleus (MN). The MN assay in P53-proficient human TK6 cells complies with OECD 487 As part of our NAMs program the MN assay can be conducted in metabolically competent Phase I and Phase II metabolism in HepaRG cells that are emerging as an in vitro toxicology model aimed as an alternative to in vivo genotoxicity assessments. Because we use flow cytometry, as opposed to the laborious traditional technique, our test is more sensitive and can be performed faster. By using human cells, our results don’t suffer from the risk that the results may suffer from interspecies differences (for example if we were using rat cells).
Use of Toxicogenomic (TgX DDI)
With TgX DDI, we can determine if there is a transcriptomal response for a subset of genes that have been determined to be predictive of genotoxicity at a given dose which is referred to as the point of departure (POD) or benchmark dose (BMD). The value of using TgX DDI is that the transcriptomic analysis may provide additional information on other safety or efficacy endpoints related to modes of action. The ScitoVation computational team toxicology expertise can extrapolate the data (including the endpoints not related to genotoxicity) to estimate Human Equivalent Exposure estimate and safety margin of exposure.
