by Scott Slattery
In May and June of this year, the annual Webinar Series on Inhalation Toxicity Testing was co-hosted by The US EPA, the PETA Science Consortium International, Syngenta, and Unilever. The nine webinars presented over three days provided an excellent look at current progress in the area of non-animal inhalation toxicity testing approaches. The talks covered the breadth of the field, addressing in vitro assay approaches, airway dosimetry modeling, and the convergence of these, as well as the prospects for applying these approaches to risk assessment. One of my focuses at ScitoVation is developing our in vitro air–liquid interface exposure capabilities for safety testing of gases, vapors, and aerosols, so it was great to hear about the latest progress and perspectives of experts in these various specialties. The webinar presentations were all recorded and are available here. I encourage you to check out the list of talks to see if there is one or more that would interest you.
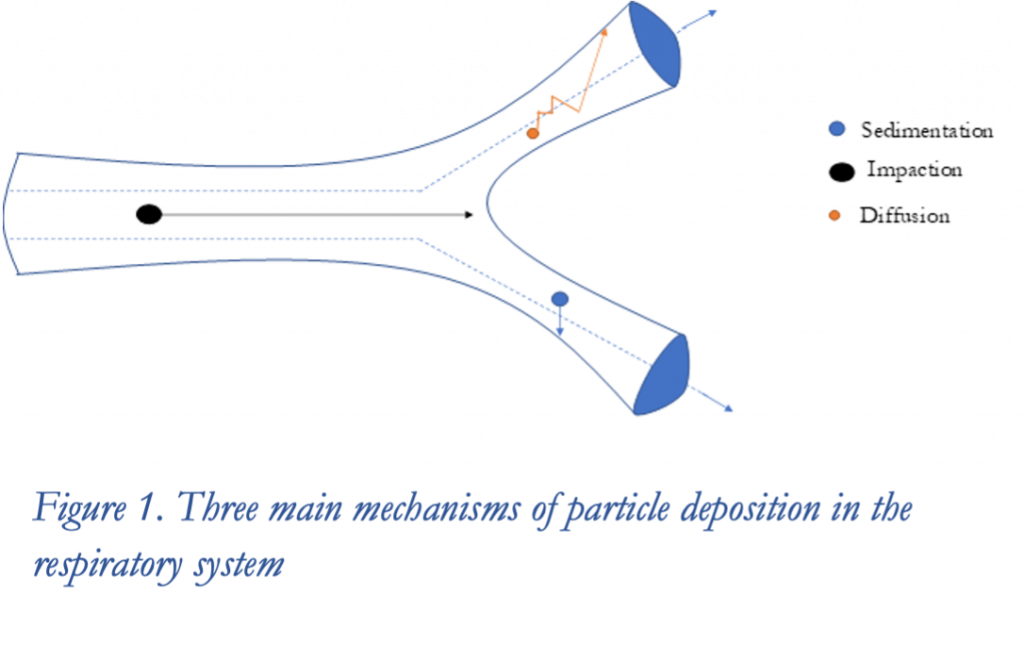
Because of our recent efforts with aerosol exposures, I was particularly interested in the first presentation of the series by Arkadiusz Kuczaj, from the University of Twente and Philip Morris International, entitled “Accurate Aerosol Dosimetry Predictions Using In Silico and In Vitro Approaches for Risk Assessment Methods” (Kuczaj 2021), and I’d like to highlight that talk in this post. Dr. Kuczaj began his presentation with a fascinating, but daunting, introduction to the complexity of aerosol dosimetry. He explained that aerosol deposition, whether it be in the airway or in an in vitro exposure system, occurs through various mechanisms (e.g., diffusion, sedimentation, and impaction) that depend upon the physical characteristics of the aerosol, including the particle density and the particle size distribution. The challenge is these physical characteristics can be extremely difficult to pin down. This is because these characteristics are not static, but rather evolve with time and with changes in their surrounding thermodynamic and physical environment. This is particularly true of aerosols composed of liquid compounds. Such compounds can transition between the liquid aerosol particle and the gas phase through condensation and evaporation with changes in pressure or temperature, and the deposited dose will be the sum of particulate deposition and absorption of vapor. Additionally, new particles can be nucleated, particles can break up into multiple smaller particles, or multiple small particles can coalesce. Particle size selection can also take place, as particles in certain size ranges can be lost from the population due to deposition or due to inertia in a bifurcating air stream. The picture is even further complicated when the aerosol is composed of mixtures of compounds with non-ideal behavior, in which case the chemical composition of the aerosol particles can change across time or space as particular species preferentially enter the gas or liquid phases. All of these forms of evolution must be considered when attempting to use physical aerosol characterization to predict deposited doses in vivo or in vitro. Although instruments exist to measure these physical aerosol characteristics, they offer only a snapshot in the evolution, usually at a point outside of the lungs or exposure system. Furthermore, as these instruments require sparse aerosols for proper measurements, tested aerosols are often diluted prior to measurement, and the dilution, of course, alters the measured characteristics.
In addition to the aerosol’s physical characteristics, other critical factors influence deposition. The morphology of the flow path (e.g., the lung morphology in vivo or the shape of an in vitro exposure system flow path) and the flow topography (which describes the variations in flow rate over time, as in breathing patterns in vivo) determine how forces act on the aerosol particles to cause deposition. Dr. Kuczaj presented the state of our understanding of these factors and suggested that our current knowledge is limited.
This thorough introduction to the numerous challenges related to aerosol dosimetry brought Dr. Kuczaj to his thesis statement “Dosimetry requires multidisciplinary and synergistic efforts.” He then went on to describe several recent efforts within his group to improve dosimetry prediction on multiple technological fronts.
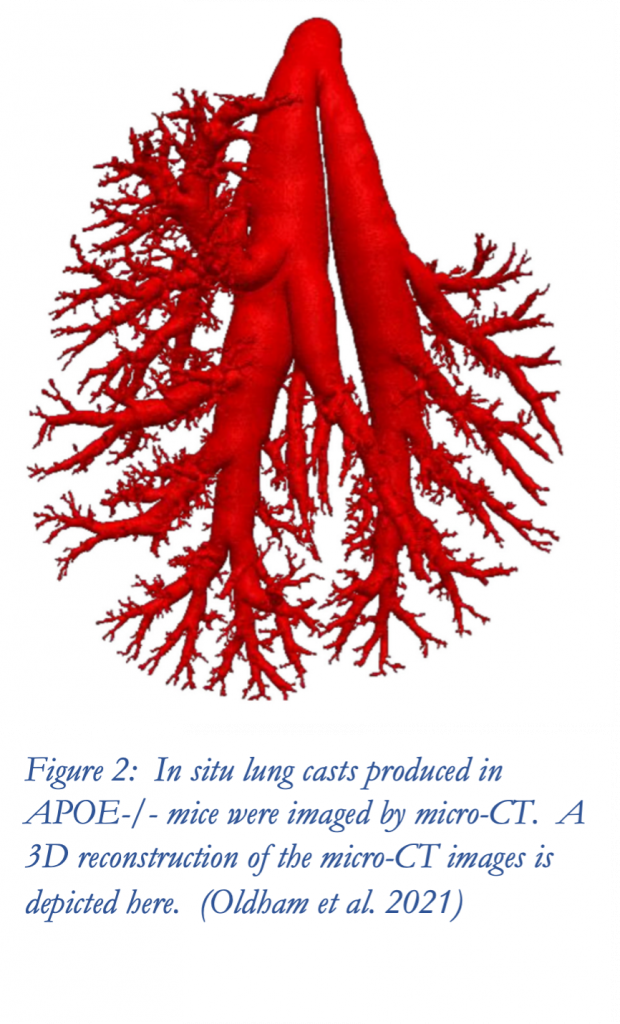
First, he described the use of in situ lung casts and micro-computed tomography (micro-CT) scans to morphometrically characterize the tracheal-bronchial airways of mice. The morphometric data extracted from these scans can be used to improve whole-lung dosimetry models, which utilize “simplified aerosol inhalation physics with relation to the geometry of the lung”.
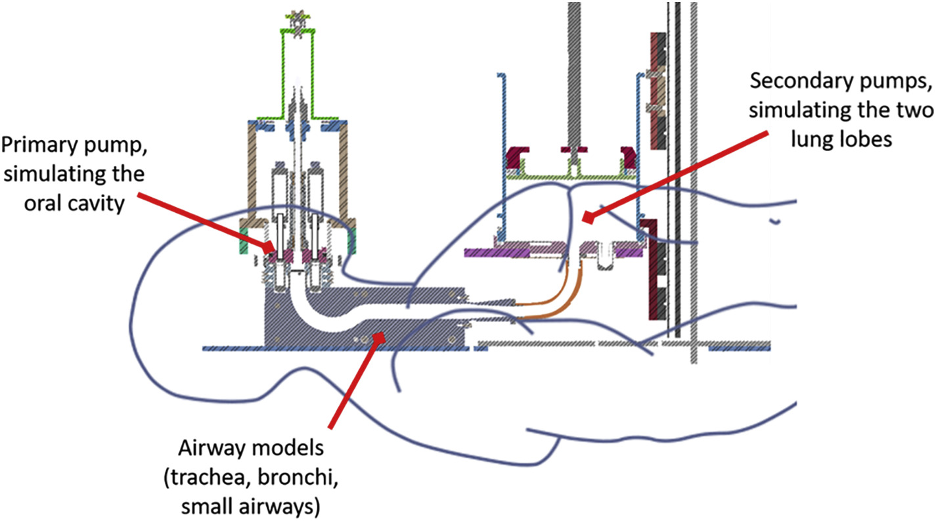
Second, Dr. Kuczaj described an in vitro exposure system, InHALES, that is based on physiologically driven processes. Unlike most other exposure systems currently in use, the InHALES system utilizes flow paths that mimic the human airway in shape and size. “Lung pumps” simulate human inhalation topography. Aerosols pass through a simulated bronchial tree before reaching the cell culture models that are being exposed. All of this is to create an in vitro system in which the aerosol evolution better replicates the aerosol evolution that takes place within the human airway, especially for complex mixture aerosols, so that the in vitro culture model is exposed to aerosols with the same physical and chemical characteristics as will be the in vivo tissues.

Thirdly, AeroSolvedTM (www.aerosolved.com), an open-source computational fluid dynamics (CFD) model based on OpenFOAM®, was presented. This code was described as simulating the generation, transport, evolution, and deposition of multispecies aerosols. While the presenter described CFD models such as this one as the gold-standard of deposition models, he noted that they are very computationally intensive and best suited for research purposes and for validation of simpler whole lung models.
Lastly, Dr. Kuczaj described physical cast models of the human upper respiratory tract that can be used to monitor aerosol evolution and deposition. The models are designed to include temperature and humidity controls that simulate the in vivo human airway, factors which affect the evolution of an aerosol as it travels down the airway. Additionally, the model can be separated into parts that can then be individually assessed for deposited aerosol quantity. Experimentation with the physical model can be paired with in silico simulations using the geometry to understand relationships between aerosol evolution and deposition.
The recording provides a thorough and interesting background and describes cutting edge work in several areas relevant to aerosol dosimetry prediction. The total recorded length is 25 minutes, but the slides are so full of information that I spent a lot of extra time pausing and rewinding to fully digest it all. There are plenty of useful references that direct the viewer to further reading on the various covered topics. If this is a field that interests you, I again encourage you to check out the recording, and the other webinars from the series while you’re at it.
Kuczaj, A. K. 2021. “Accurate aerosol dosimetry predictions using in silico and in vitro approaches for risk assessment methods.” In 2021 Webinar Series on Inhalation Toxicity Testing. US Environmental Protection Agency, Unilever, Syngenta, & PETA Science Consortion International.
Oldham, M. J., F. Lucci, C. Foong, D. Yeo, B. Asgharian, S. Cockram, S. Luke, J. Chua, J. Hoeng, M. C. Peitsch, and A. K. Kuczaj. 2021. ‘Use of micro-CT to determine tracheobronchial airway geometries in three strains of mice used in inhalation toxicology as disease models’, The Anatomical Record, 2021: 1-18. https://doi.org/10.1002/ar.24596
Steiner, S., P. Herve, C. Pak, S. Majeed, A. Sandoz, A. Kuczaj, and J. Hoeng. 2020. ‘Development and testing of a new-generation aerosol exposure system: The independent holistic air-liquid exposure system (InHALES)’, Toxicol In Vitro, 67: 104909.
