Predicting Equivalent Dose in Humans
Physiologically Based Pharmacokinetic (PBPK) Modeling is a predictive tool to estimate how compounds are absorbed, distributed, metabolized, and excreted in a body. PBPK models provide a mechanistic approach to study and predict the disposition of chemicals or drugs based on physiologic and anatomic characteristics, as well as the physical and chemical properties of a given chemical or drug. These models are used in the field of toxicology for the prediction of human and animal exposures to environmental toxins and in the drug industry to help in drug development.
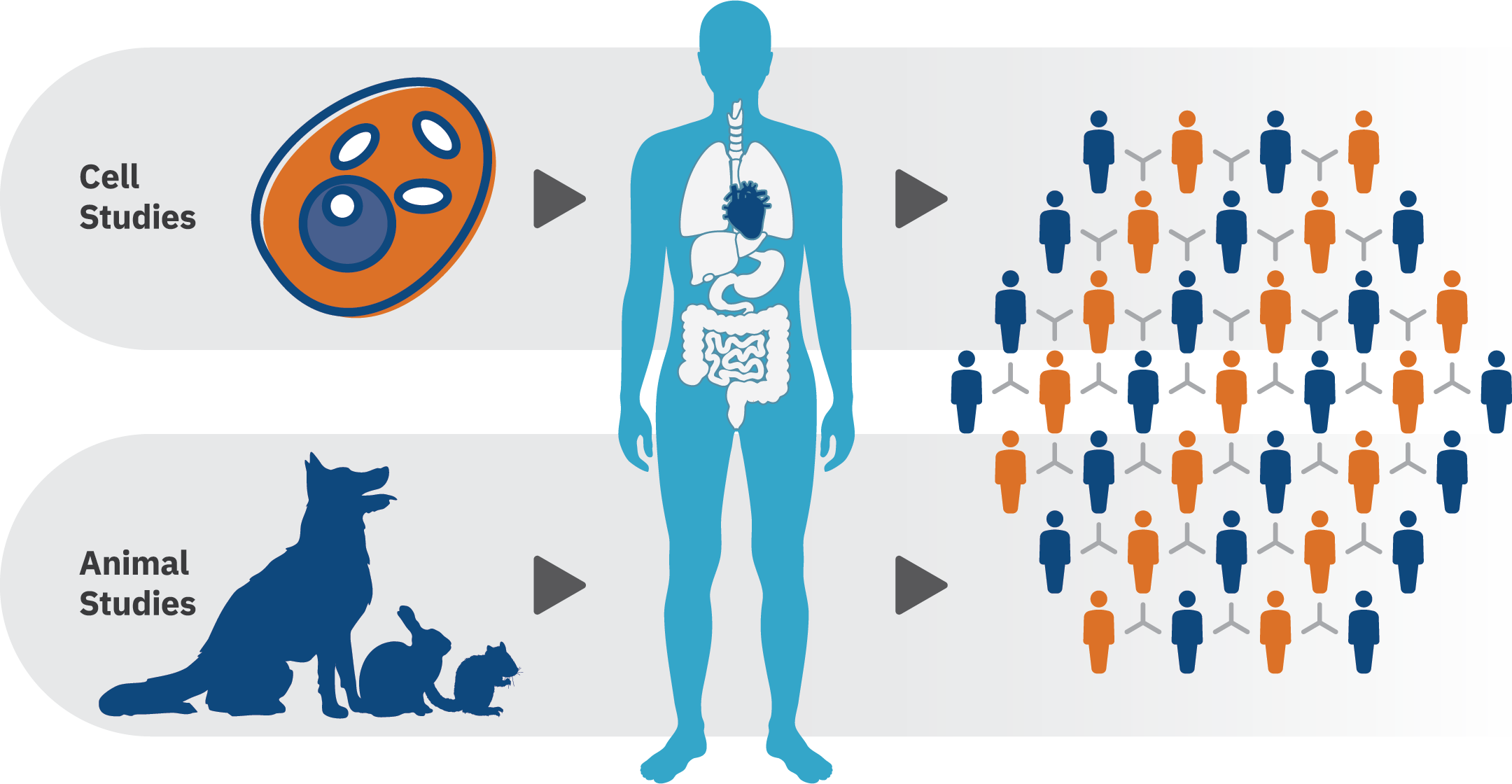
PBPK modeling helps our clients:
- Predict exposure from one species to another, for example: you have a dose of exposure giving a certain concentration in blood in rats and you would like to estimate the dose of exposure giving the same blood concentration in a human.
- Capture human variability by including monte Carlo analysis in the PBPK model.
- Estimate a response from varying exposure conditions and across life-stages or vulnerable populations, such as children, the elderly, or those working in specific occupations. In one of our projects that was submitted for regulatory purposes, we addressed the concern for age-related sensitivity to pyrethroids with life-stage physiologically based pharmacokinetic (PBPK) modeling supported by in vitro to in vivo extrapolation (IVIVE) to predict age-dependent changes in target tissue exposure to pyrethroids.
- Predict concentration in blood or tissue from dermal exposure using oral exposure data, called route-to-route extrapolation.
- Conduct high-dose to low-dose extrapolation to predict concentration in blood and tissue for exposure at different doses. Most of the time, animal experiments are conducted at high dose to measure effects. PBPK models can extrapolate the concentration in blood or at target organs at lower doses.
- Estimate population daily exposure intakes that are consistent with blood or urine measures found in biomonitoring surveys (reverse dosimetry).
- Predict both dose-response and time-course for the development of adverse effects, when coupled with pharmacodynamics (PD) models.
Why clients use ScitoVation to address dosimetry:
- We are pioneers in the field and we developed our own open source PBPK tool, PLETHEM (Population Life-course exposure to health effects model). Because PLETHEM was developed under a memorandum of understanding with the US EPA, submissions to regulatory bodies are likely better received when the computations are performed using PLETHEM.
- We are a multidisciplinary team developing models that best fit the client’s needs. With the reduction of animal testing, most PBPK model parameters are derived from in vitro testing or in silico tools (metabolism, binding in plasma, partition coefficient). Using our own laboratory to generate the data needed as input for our models, results in efficiency for our clients.
- We developed dozens of PBPK models, some of which are used in regulatory decisions (EPA, ECHA): Clients benefit from our development experience and regulator interaction. This enables us to provide regulators the information in an accepted format which increases the likelihood of approval and decreases time for a decision.

Case Study: Investigating Metabolism Impact Of Internal Butylparaben Concentration
