by Sage Corzine
If you got pumped up about NAMs in a toxicology webinar, tried to find more information on Google, and got redirected to the North American Menopause Society, you are not alone. NAMs or New Approach Methodologies are a vital and growing part of chemical hazard and risk assessment research. Here are a few fun facts that Google doesn’t want you to know.

A Brief History of NAMs
In 2016, the Lautenberg Chemical Safety act was passed, amending the Toxic Substances Control Act of 1976 and added two very important directives, which will be evaluated every 5 years:
- “Reduce and replace, to the extent practicable and scientifically justified, the use of vertebrate animals in the testing of chemical substances or mixtures;
- Promote the development and timely incorporation of alternative test methods or strategies that do not require new vertebrate animal testing”.[1]
In short, if there is a proven method to get the same crucial data on chemical safety without harming any animal, by law we should use it. With modern technology we can get a plethora of data from human and animal cells. The challenge is proving that this data can be used to consistently predict effects on humans. At ScitoVation, we help meet that challenge by developing and testing NAMs.
Important Terms to Know
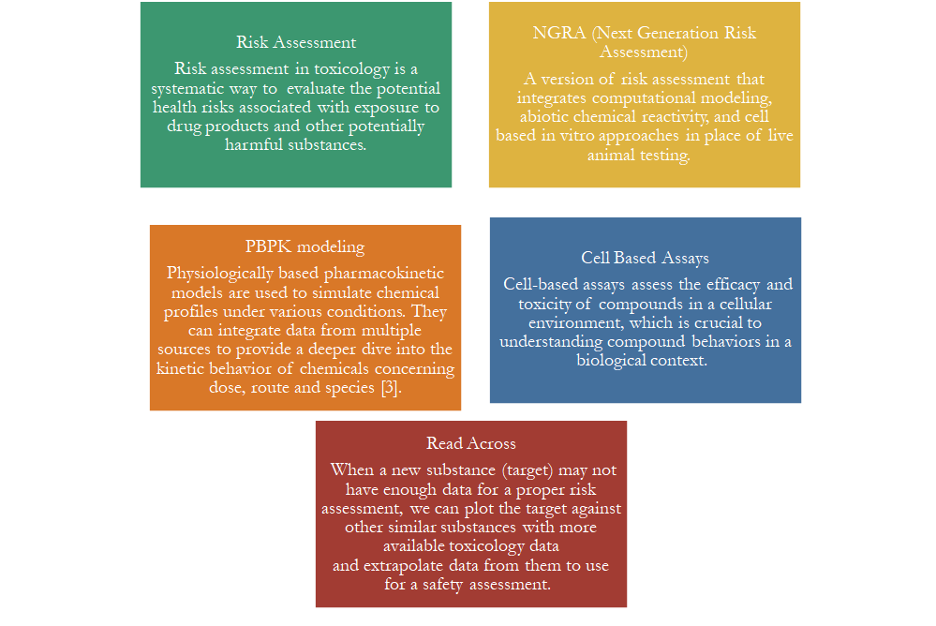
Benefits and Challenges of NAMs
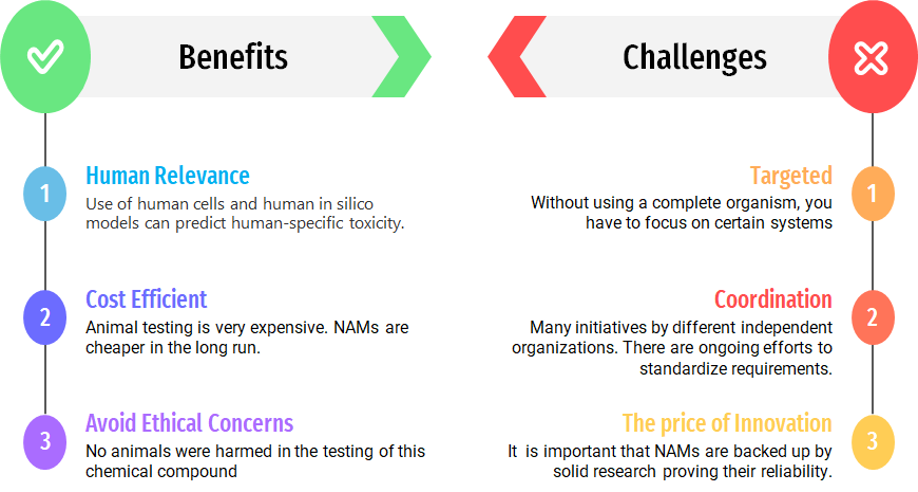
Understanding the benefits of NAMs not only help you use cutting edge technology, but also save money and avoid ethical concerns. If you have any questions about NAMs or how NAMs can be applied to an upcoming project, reach out to us. We are alway happy to talk.
2. Parish ST, Aschner M, Casey W, Corvaro M, Embry MR, Fitzpatrick SF, Kidd D, Kleinstreuer NC, Lima BS, Settivari RS, Wolf DC, Yamazaki D, Boobis A. An evaluation framework for new approach methodologies (NAMs) for human health safety assessment. Regul Toxicol Pharmacol. 2020 Apr;112:104592. doi: 10.1016/j.yrtph.2020.104592.
3. Espié P, Tytgat D, Sargentini-Maier ML, Poggesi I, Watelet JB. Physiologically based pharmacokinetics (PBPK). Drug Metab Rev. 2009;41(3):391-407. doi: 10.1080/10837450902891360.
