Client problem
Parabens are widely used as preservatives in products in pharmaceutical, food, and cosmetics. As the general population is exposed every day to a variety of personal care products, risk assessment is necessary to assure safe use of these products. At this time, there is no information showing that parabens that are used in cosmetics influence human health. However, mostly based on non-guideline academia studies, parabens are suspected to affect hormone function in animals. There is some evidence of differences in butylparaben metabolism between humans and rats after dermal exposure (SCCS). This difference is potentially important because the availability of un-metabolized butylparaben is expected to determine their biological activity and toxicity. Here, we investigate the extent to which species differences in metabolism impact internal butylparaben concentration.
ScitoVation Solution
A PBPK model for butylparaben developed by Campbell et al. (2015) was refined and used to estimate internal exposure in rats and humans for dermal, oral, and IV routes of dosing or exposure. These rat and human models were used in a margin of internal exposure (MOIE)-based risk assessment. Additionally, these models provided a better overall understanding of toxicokinetic differences between dosing or exposure routes and across species to address some previous concerns of the scientific committee on consumer safety (SCCS) in their 2013 opinion.
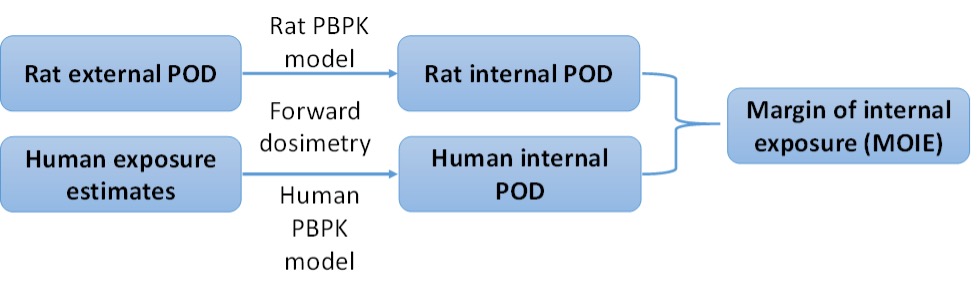
Figure 1. Steps for MOIE calculation
A MOIE differs from a traditional margin of exposure (MOE) in that it is calculated as the ratio of a measure of internal exposure, such as blood concentration or target-tissue dose, rather than a measure of external exposure concentration or ingested dose (Bessems et al. 2017). The ability to rely on a measure of internal rather than external exposure reduces the uncertainty in the risk assessment by incorporating chemical-specific information on the uptake, distribution, metabolism and excretion of the chemical in both the experimental animal and the human (Clewell et al. 2008).
Results
We derived a MOIE based on PBPK simulations of an experimental animal dosing scenario and hypothesized human dermal exposure scenarios. The modelling approach predicts the internal dose metrics (blood AUC and Cmax) for butylparaben that result from the oral dosing of rats, from human dermal exposure. This work is intended to support the butylparaben risk assessment.
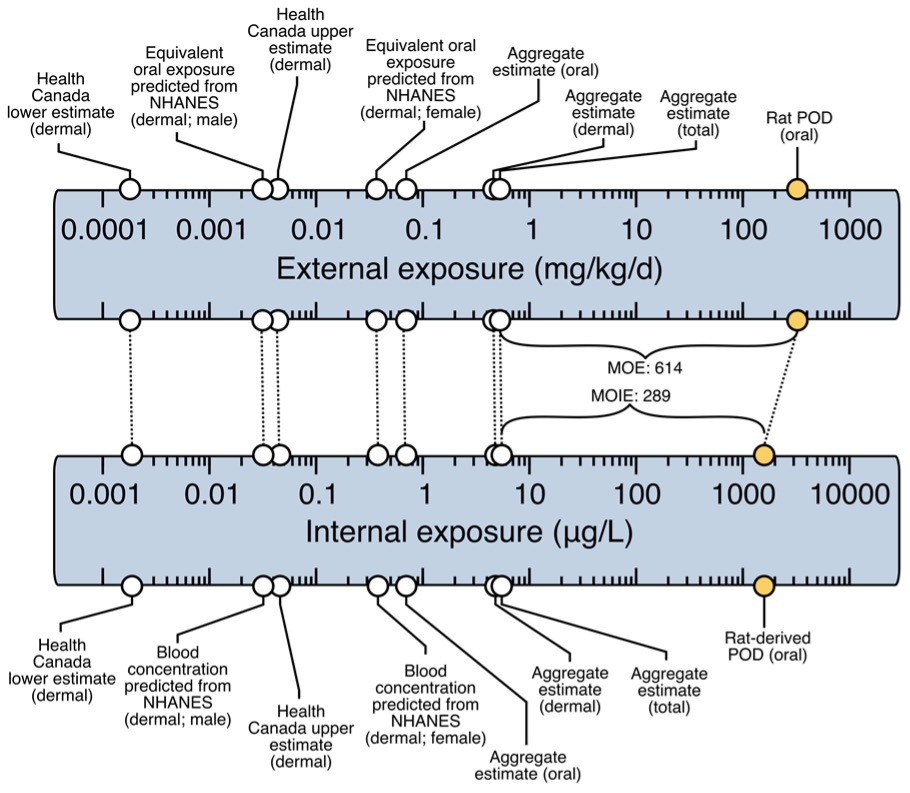
Figure 2. MOIE schematic representation.
The upper scale represents the external exposure while the lower scale represents the internal blood concentration. The yellow points represent the external dose representative of an oral rat POD and the equivalent blood concentration at the POD. This internal value is then compared to internal blood concentrations following aggregate scenario exposures or to biomonitoring data that are representative of the exposure to butylparaben in the population. In this figure, we have exposure estimates in the Canadian and US population that were calculated by reverse dosimetry with the PBPK model based on urine data in the general population. These external exposure estimates were then used in the PBPK model to derive a blood concentration that can be used to calculate the MOIE.
This MOE for butylparaben is higher compared to the usual minimal MOE/MOS value of 100. The MOE is usually defined as the ratio between the external dose in the animal study and the human scenario specific external exposure (both in mg/kg bw). The traditional MOE approach does not consider physiological animal to human differences that influence toxicokinetics or differences in absorption (dermal or oral). In this approach, we derived an MOIE which is based on the ratio of the internal exposure dose metrics (Blood Cmax and AUC). The MOIEs reported for butylparaben are lower than the traditional MOE. This indicates that the use of the MOIE is a more conservative approach and more realistic as it takes into account differences in absorption, distribution, metabolism and excretion between species. It is specifically important for butylparaben when it has been shown that there is a difference in metabolism between rats and humans.
