September 25, 2022
NAM-Based Prediction Of Point-Of-Contact Toxicity In The Lung: A Case Example With 1,3-Dichloropropene
Purpose
- Chemicals can be inhaled in different physical states (gases, and vapors) that can cause a wide range of adverse health effects, ranging from irritation at the portal of entry (respiratory tract) to systemic diseases in the body.
- Inhaled chemicals interface along the airway epithelium and interact with lung tissues. The airway epithelium is diverse and composed of different cell types in different proportions along various regions of the airway. Each distinct lung tissue may have distinct sensitivity to toxins, both because the unique physiological characteristics of each tissue may create differential toxicodynamic responses, as well as because the different localization of each tissue within the airway may cause differential exposure after inhalation of an airborne toxin.
- The objective of ScitoVation’s work was to assess whether in vivo points of departure (PODs) for point-of-contact inhalation toxicants can be predicted by an approach that combines in vitro exposures of organotypic cultures to airborne test compounds and in silico modeling of airway dosimetry. In vitro to in vivo extrapolation (IVIVE) converts chemical concentrations that are active in experimental in vitro systems into equivalent in vivo exposure levels via reverse dosimetry with pharmacokinetic (PK) modeling.
- IVIVE is a major integral component in new alternative methods (NAM) based risk assessment.
Method
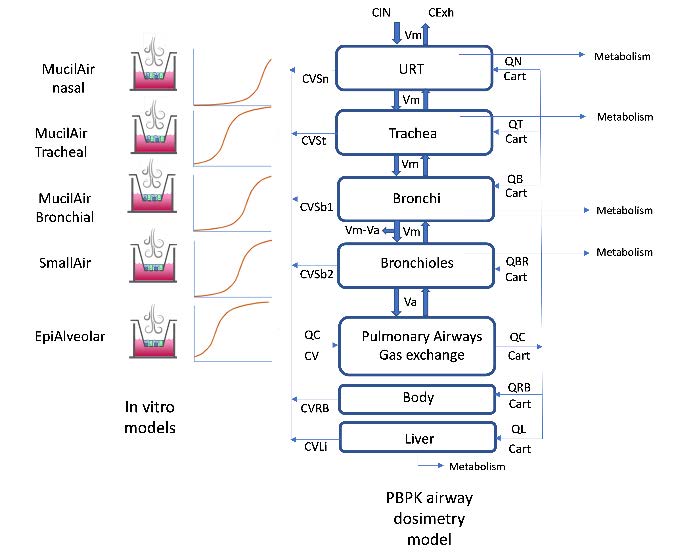
- Five human-derived, differentiated, in vitro airway epithelial cell culture models (MucilAir of nasal, tracheal, or bronchial origin, SmallAir, and EpiAlveolar) representing five regions of the
airways (epithelium—nasal, tracheal, bronchial, bronchiolar, and alveolar) were exposed to a known toxic vapor, 1,3-dichloropropene (1,3-DCP). - We monitored the toxicity in these cultures 24 hours after acute exposure using an assay for transepithelial conductance (for epithelial barrier integrity) and assay for cytotoxicity (for lactate dehydrogenase – LDH release).
- Finally, we have developed an airway dosimetry computer model for 1,3-DCP vapor to predict in vivo external exposure
scenarios that would reproduce toxic local tissue concentrations as determined by in vitro experiments.
Results
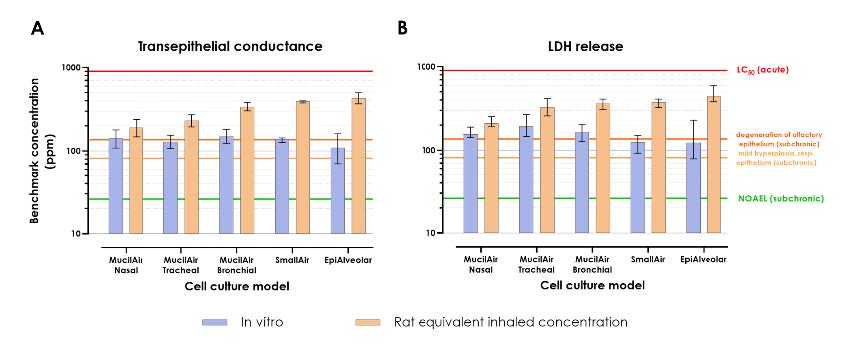
- Measured in vitro points of departure (PoDs) for all tested cell culture models were similar.
- Calculated rat equivalent inhaled concentrations varied according to position of the modeled
tissue within the airway, with nasal respiratory tissue being the most proximal and most sensitive tissue, and alveolar epithelium being the most distal and least sensitive tissue. - These predictions are qualitatively in accordance
with empirically determined in vivo PoDs.
Client value
- This case study has implemented a NAM-based chemical risk assessment approach that uses in vitro and in silico methods to account for pharmacodynamic and pharmacokinetic factors of in vivo toxicity. This study will contribute to the body of similar work demonstrating the potential of NAMs to contribute to more efficient, economical, ethical, and effective chemical risk assessment.
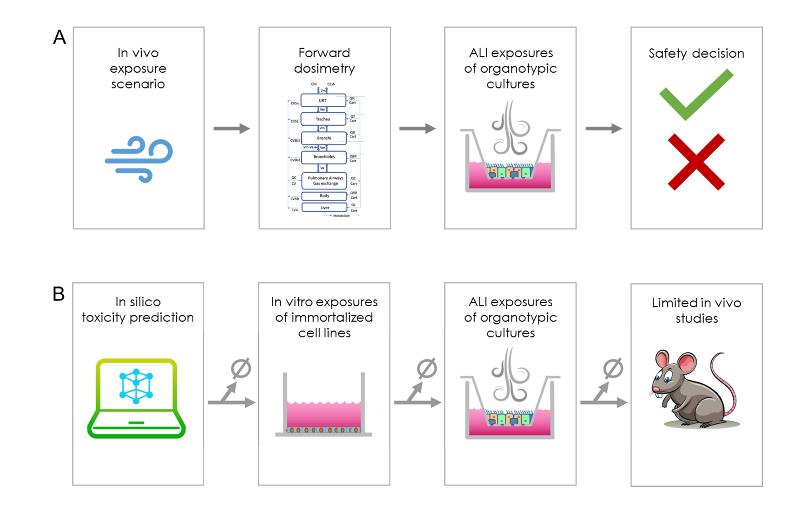
- The flexibility of the present framework can inform NAM-based testing in a variety of decision
contexts (Figure 2). In the case of exposure to a new volatile chemical (A), a decision must be made regarding the risk. For example, forward airway dosimetry modeling could be applied to extrapolate that inhaled concentration to luminal concentrations at regions throughout the airway. Those predicted luminal concentrations could then be tested in vitro on
site-specific differentiated airway epithelial cultures. A finding of acute in vitro toxicity at any of those luminal concentrations would caution against that use of that compound. - In the case of multiple volatile compounds, chemicals can be prioritized to choose the safest option for further development. A tiered testing approach would efficiently help the decision process (B). First, purely computational approaches, such as QSAR modeling, could be applied to reject compounds with a high probability of toxicity. Next, in vitro exposures using an air-liquid interface (ALI) with affordable immortalized cell lines may be sufficient to reject a subset of compounds with relatively high toxicity. For the remainder, testing in differentiated ALI cultures followed by IVIVE to determine an in human equivalent dose would provide more accurate predictions of relative toxicity and allow for further down-selection. Lastly, limited in vivo testing could be performed as needed only on the final subset of compounds.